Method for preparing medicament sustained-release material
A drug preparation and sustained-release technology, applied in the field of preparation of biodegradable polymer drug sustained-release materials, can solve the problems of flexible and effective control of drug administration schemes, difficulty in meeting the release requirements of drugs with different solubility properties, and achieve broad industrialization and market Prospects, stable product quality indicators, and the effect of improving phase morphology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060]A polycaprolactone (matrix) / polyethylene oxide (disperse phase) drug sustained-release composite material comprises the following components and contents in parts by weight:
[0061]
[0062] Explanation: the weight of cephalexin (being a drug) is 1% of the total weight of the biodegradable polymer matrix and the dispersed phase.
[0063] The first step, at first prepare raw materials by above-mentioned components;
[0064] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0065] In the third step, the dried cephalexin, polycaprolactone and polyethylene oxide obtained in the second step are placed in a high mixer and premixed for 5 minutes at a speed of 100 rpm to obtain a drug and polymer premix , and dry the premixed composite particles in a vacuum oven at 50 °C for 3 hours.
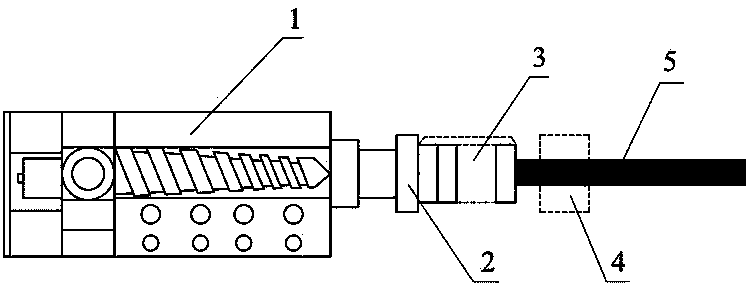
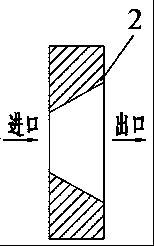
[0066] In the fourth step, the drug and the polymer premix obtained in the third step are put into figure 1 The single-screw extruder 1 of the integrated biaxia...
Embodiment 2
[0071] A polylactic acid (matrix) / polyethylene oxide (dispersed phase) drug sustained-release composite material comprises the following components and their contents in parts by weight:
[0072]
[0073] Explanation: the weight of diclofenac sodium (being a medicine) is 5% of the total weight of the biodegradable polymer matrix and the dispersed phase.
[0074] The first step, at first prepare raw materials by above-mentioned components;
[0075] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0076] In the third step, the dried diclofenac sodium, polylactic acid and polyethylene oxide obtained in the second step are premixed together in a high-speed mixer for 5 minutes at a speed of 100 rpm to obtain a drug and polymer premix. Then put the obtained drug and polymer premix into a twin-screw extruder for melt blending, extrusion, and granulation to obtain premixed composite granules of polylactic acid / polyethylene oxide / diclofenac sodium, and ...
Embodiment 3
[0081] A polycaprolactone-polylactic acid (matrix) / polyethylene oxide (dispersed phase) drug sustained-release composite material comprises the following components and contents in parts by weight:
[0082]
[0083] Explanation: the weight of ibuprofen cephalexin (being medicine) is 20% of the total weight of the biodegradable polymer matrix 1-2 and the dispersed phase.
[0084] The first step, at first prepare raw materials by above-mentioned components;
[0085] In the second step, the drug is dried in a vacuum oven at 60°C for 6 hours;
[0086] In the third step, the dried ibuprofen, polycaprolactone, and polylactic acid obtained in the second step are put into a twin-screw extruder for melt blending, extrusion, and granulation to obtain premixed granules of medicine and polymer. The mixed composite particles were dried in a vacuum oven at 50 °C for 3 hours, and the temperatures of the feeding port, conveying section, melting section, homogenizing section and die of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com