Cefixime chewable tablet and preparation method thereof
A technology for cefixime and chewable tablets, which is applied to the field of cefixime chewable tablets and its preparation, can solve the problems of limited use of tablets, low bioavailability, low physical strength, etc., and achieves elimination of specific odor and bioavailability. High, curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
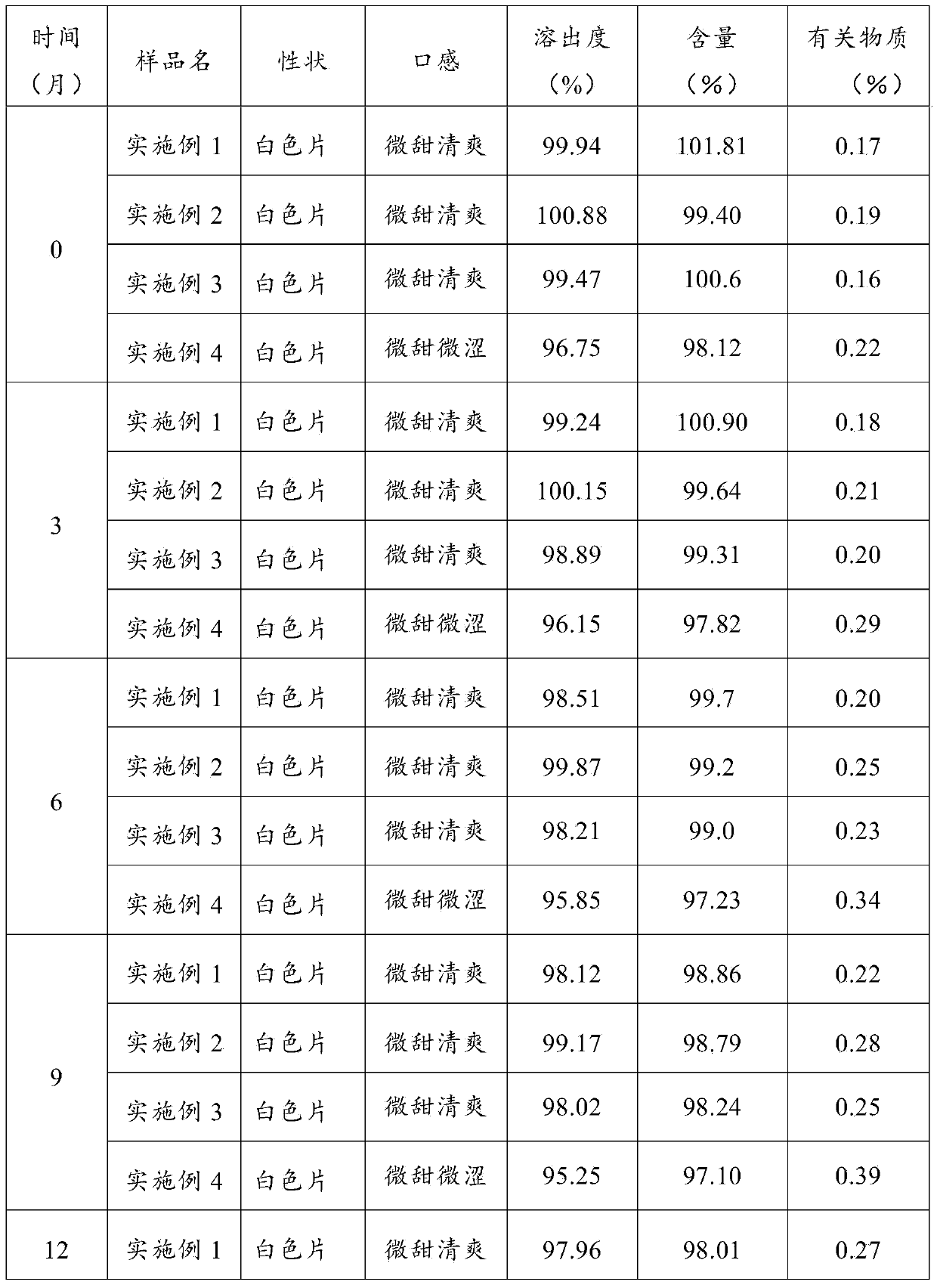
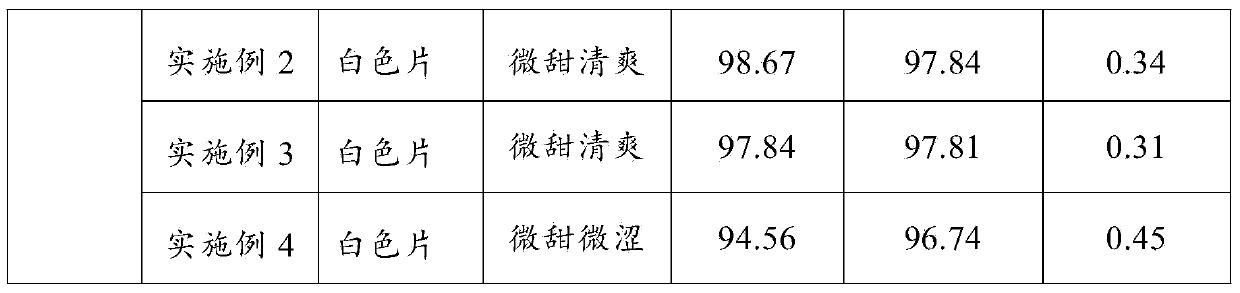
Embodiment 1
[0037] Prescription: 10000 tablets
[0038] Cefixime Anhydrous 1000g Mannitol 1600g
[0039] Microcrystalline cellulose 150g Pregelatinized starch 70g
[0040] Sweet orange flavor 10g Magnesium stearate 50g
[0041] Preparation:
[0042] (1) Cefixime passes through a 100-mesh sieve, and the auxiliary materials pass through a 80-mesh sieve respectively, and set aside;
[0043] (2) Mix the prescribed amount of cefixime anhydrate, mannitol, microcrystalline cellulose, pregelatinized starch, and sweet orange flavor in a mixer;
[0044] (3) Add magnesium stearate to step (2) and mix for 25 minutes. Set the tableting environment temperature to 25°C and the humidity to 60% to press the tablet. The tableting pressure is controlled at 40KN, and the speed is 12,000 tablets per hour. Tablets to prepare cefixime chewable tablets.
Embodiment 2
[0046] Prescription: 10000 tablets
[0047] Cefixime Anhydrous 1100g Mannitol 1500g
[0048] Microcrystalline cellulose 120g Pregelatinized starch 60g
[0049] Sweet orange flavor 15g Magnesium stearate 60g
[0050] Preparation:
[0051] (1) Cefixime passes through a 100-mesh sieve, and the auxiliary materials pass through a 80-mesh sieve respectively, and set aside;
[0052] (2) Mix the prescribed amount of cefixime anhydrate, mannitol, microcrystalline cellulose, pregelatinized starch, and sweet orange flavor in a mixer;
[0053] (3) Add magnesium stearate to step (2) and mix for 25 minutes. Set the tabletting environment temperature at 25°C and the humidity at 55% to press the tablet. The tableting pressure is controlled at 35KN, and the speed is 12,500 tablets per hour. Tablets were prepared to prepare cefixime chewable tablets.
Embodiment 3
[0055] Prescription: 10000 tablets
[0056]Cefixime Anhydrous 1200g Mannitol 1700g
[0057] Microcrystalline cellulose 170g Pregelatinized starch 80g
[0058] Sweet orange flavor 20g Magnesium stearate 70g
[0059] Preparation:
[0060] (1) Cefixime passes through a 100-mesh sieve, and the auxiliary materials pass through a 80-mesh sieve respectively, and set aside;
[0061] (2) Mix the prescribed amount of cefixime anhydrate, mannitol, microcrystalline cellulose, pregelatinized starch, and sweet orange flavor in a mixer;
[0062] (3) Add magnesium stearate to step (2) and mix for 30 minutes, set the tableting environment temperature to 30°C, and press the tablet under the conditions of humidity 65%, the tableting pressure is controlled at 45KN, and the speed is 13,000 tablets per hour. Tablets were prepared to prepare cefixime chewable tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com