Method for increasing proportion of paradichlorobenzene in benzene chlorination product
A technology for chlorination of p-dichlorobenzene and benzene, applied in the field of benzene chlorination, can solve the problems of high price, easy poisoning and inactivation, and limited industrial application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
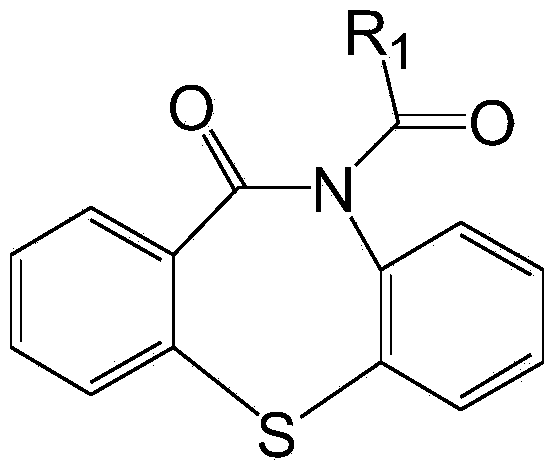
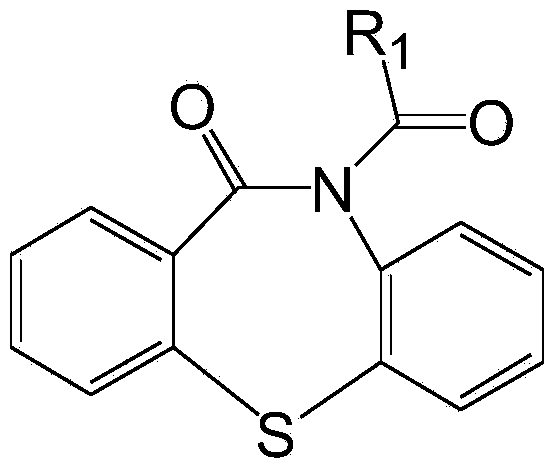
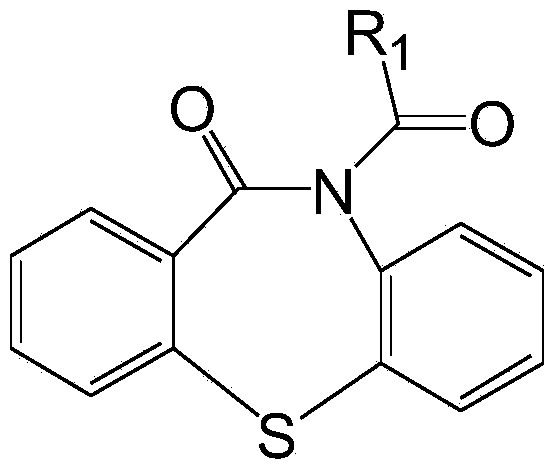
[0020] Preparation of cocatalyst dibenzothiazepine-N-carbonyl chloride: Weigh 68.10g dibenzo[b,f][1,4]thiazepine-11-[10H]ketone, 26.10g pyridine , 300ml of toluene, sequentially added to a 2000ml three-necked flask. Another 32.64g of triphosgene was dissolved in 300ml of toluene and added to the constant pressure dropping funnel. Add the triphosgene solution dropwise to the reaction flask under stirring at room temperature, and finish the drop within 3 hours. Continue stirring for 1 hour and then raise the temperature to reflux for 1 hour, and then change to distillation to recover most of the toluene. Stop heating, after cooling and crystallization, filter. The solid was washed twice with ice water at 0°C, and dried in a vacuum oven at 35°C to obtain dibenzothiazepine-N-carbonyl chloride.
Embodiment 2
[0022] Weigh about 30g of benzene, 35mg of iron powder, and 35mg of dibenzothiazepine-N-carbonyl chloride, and evenly and slowly pass chlorine gas under stirring for 4 hours. The temperature range of the whole process is controlled at 60-70°C. After the reaction, filter, Wash with water, dry over anhydrous sodium sulfate, and filter. Sampling was carried out for gas chromatography detection. The conversion rate of benzene was 99.8%, the conversion rate of chlorobenzene was 92.7%, the content ratio of p-dichlorobenzene and o-dichlorochlorobenzene was about 4.8, and the content of m-dichlorotoluene was 0.36%.
Embodiment 3
[0024] Weigh about 30g of benzene, 20mg of iron powder, and 20mg of dibenzothiazepine hydroxamic acid, and evenly and slowly pass chlorine gas for 5.5 hours under stirring. The temperature range of the whole process is controlled at 50-60°C. After the reaction, filter and wash with water. Dry over anhydrous sodium sulfate and filter. Sampling was carried out for gas chromatography detection. The conversion rate of benzene was 99.8%, the conversion rate of chlorobenzene was 90.0%, the content ratio of p-dichlorobenzene and o-dichlorobenzene was about 4.7, and the content of m-dichlorobenzene was 0.20%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com