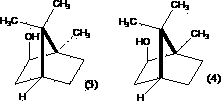
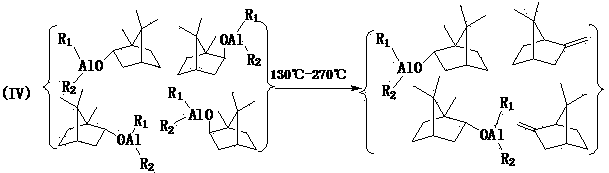
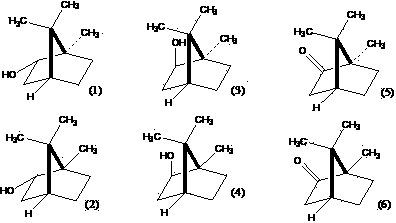
Method for preparing high-purity borneol from camphor, camphor reduction product and borneol
A high-purity, camphor-based technology, used in the fields of chemistry and medicine, can solve problems such as difficult industrialization, pollution, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Isopropanol is an example,
[0068] Take 4g aluminum powder (AR) and add 4g anhydrous AlCl 3 (AR), mix quickly, add 200ml of isopropanol (AR), under airtight, reflux in a water bath for 12h, add 100g of borneol, in a water bath until the borneol is dissolved, shake well to form a homogeneous solution. Distill about 170 ml -180 ml of isopropanol in a gas bath at 120°C. Under airtight, react at 150°C for 15h in a gas bath, add water, and after heating for 2h, pass into steam distillation, condense, and the distillate first comes out of a liquid containing camphene and a small amount of camphene. The latter mainly contains borneol crystalline solid and a small amount of camphene. A small amount of camphene was heated to dissolve borneol, and then sublimated at 170°C-180°C to obtain 40.2g. The purity of borneol was determined by GC to be 96.8%. Based on borneol, the yield of borneol was 40.2%.
Embodiment 2
[0070] Take 4g aluminum powder (AR) and add 4g anhydrous AlCl 3 (AR), mix quickly, add 200ml of isopropanol (AR), under airtight, reflux in a water bath for 12h, add 100g of borneol, in a water bath until the borneol is dissolved, shake well to form a homogeneous solution. Distill about 170 ml -180 ml of isopropanol in a gas bath at 120°C. Under airtight, react at 150°C for 15h in a gas bath, add water, and after heating for 2h, pass into steam distillation, condense, and the distillate first comes out of a liquid containing camphene and a small amount of camphene. The latter mainly contains borneol crystalline solid and a small amount of camphene. Add a small amount of camphene and 4 times the amount of borneol to dissolve in cyclohexane, divide the upper layer solution, dry with anhydrous sodium sulfate, add 200ml DMSO (AR), evenly, place it at 10℃ for 3-4h, divide the lower layer solution Or siphon the lower layer solution, dilute with water, filter, and crystallize it once...
Embodiment 3
[0072] Take 4g aluminum powder (AR) and add 4g anhydrous AlCl 3 (AR), mix quickly, add 200ml of isopropanol (AR), under airtight, reflux in a water bath for 12h, add 100g of borneol, in a water bath until the borneol is dissolved, shake well to form a homogeneous solution. Distill about 170ml -180ml of isopropanol in a gas bath at 120°C. Under airtight, react at 150℃ for 15h in a gas bath, add water, heat for 2h, pass into steam distillation, condense, the distillate first comes out of the liquid containing camphene and a small amount of camphene, using 4 times the amount of cyclohexane The alkane is dissolved and dried with anhydrous sodium sulfate to obtain sample solution (I). The latter mainly contains borneol crystalline solid and a small amount of camphene. Add a small amount of camphene and 4 times the amount of borneol to dissolve in cyclohexane, divide the upper solution, and dry with anhydrous sodium sulfate to obtain sample solution (II). Take 350g of column chroma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com