Substitution and chemistry deposition compound preparation method for nano silver coated copper powder
A chemical deposition and nano-silver technology, which is applied in the field of nano-silver-coated copper powder, can solve problems such as complex processes, and achieve the effect of simplifying the synthesis process and reducing costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
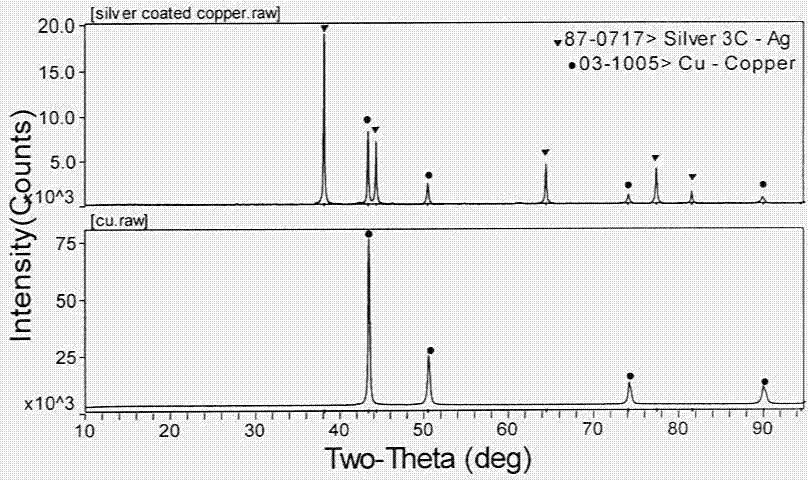
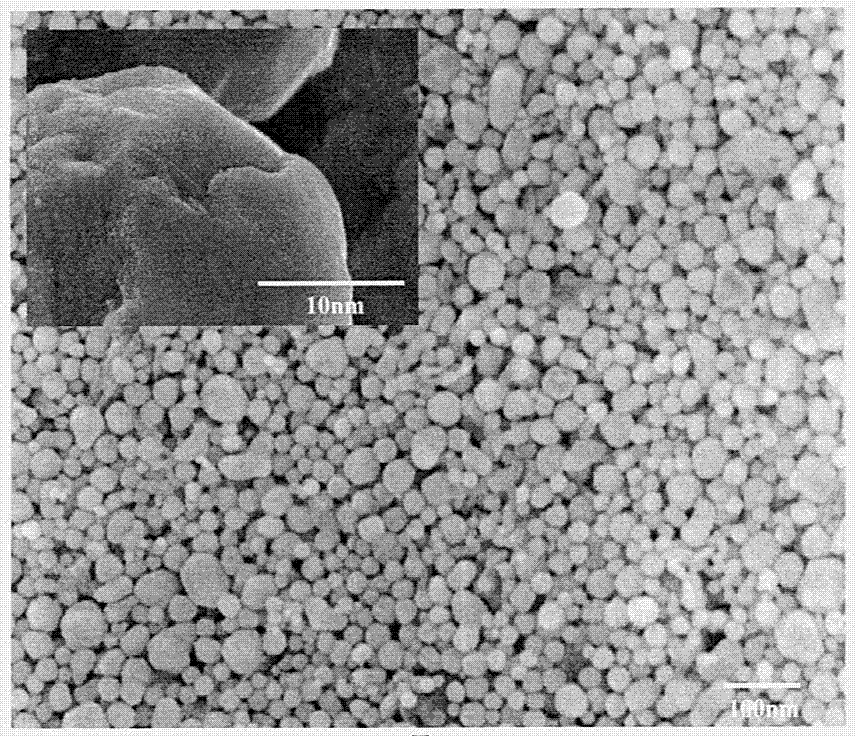
Image
Examples
Embodiment 1
[0027] Polyvinylpyrrolidone (5g) and copper sulfate (0.8g, 5mmol) were dissolved in 500ml of deionized water, adjusted to pH=12 with concentrated ammonia water to obtain solution I, polyvinylpyrrolidone (5g) and 80% hydrazine hydrate (3.125g , 0.05mol) was dissolved in 500ml deionized water to obtain solution II, and solution II was slowly added dropwise to solution I under anaerobic conditions, the reaction temperature was 15°C, magnetic stirring, 1h dropwise addition was completed, and the reaction was continued for 3h to obtain purple-red copper powder suspension. Dissolve polyvinylpyrrolidone (25g) and silver nitrate (1.7g, 0.01mol) in 500ml deionized water, adjust pH=10 with concentrated ammonia water to obtain silver ammonia solution III, and slowly drop solution III into the above copper under anaerobic conditions In the powder suspension, inject 30% formaldehyde (0.5g, 5mmol) into the reaction system after reacting for 2h, continue reacting for 2h, the solution turns f...
Embodiment 2
[0029] Hexadecyltrimethylammonium bromide (15g) and cupric chloride (1.35g, 0.01mol) were dissolved in 500ml deionized water, and adjusted to pH=9 with concentrated ammonia water to obtain cuproammonia solution I. Alkyltrimethylammonium bromide (15g) and 80% hydrazine hydrate (12.5g, 0.2mol) were dissolved in 500ml deionized water to obtain reducing solution II, and solution II was slowly added dropwise to solution I under anaerobic conditions, The reaction temperature was 45° C., magnetic stirring was performed, and the dropwise addition was completed after 2 hours, and the reaction was continued for 2 hours to obtain a purple-red copper powder suspension. Add cetyltrimethylammonium bromide (15g) and silver oxide (2.32g, 0.01mol) into 500ml deionized water, adjust pH=11 with concentrated ammonia water to obtain silver ammonia solution III, and dissolve the solution under anaerobic conditions III was slowly dropped into the above-mentioned copper powder suspension, and after 1...
Embodiment 3
[0031]Sodium dodecylsulfonate (15g) and copper nitrate (1.88g, 0.01mol) were dissolved in 500ml of deionized water, adjusted to pH=10 with concentrated ammonia water to obtain cuproammonia solution I, and sodium dodecylsulfonate (15g) and 80% hydrazine hydrate (18.75g, 0.3mol) were dissolved in 500ml deionized water to obtain reducing solution II, and solution II was slowly added dropwise to solution I under anaerobic conditions, the reaction temperature was 35 ° C, magnetic stirring, After 2 hours of dropwise addition, the reaction was continued for another 2 hours to obtain a purple red copper powder suspension. Add sodium dodecylsulfonate (15g) and silver oxide (4.64g, 0.02mol) into 500ml of deionized water, adjust the pH=11.5 with concentrated ammonia water to obtain silver ammonia solution III, slowly drop solution III under anaerobic conditions into the above-mentioned copper powder suspension, react for 2 hours, inject 30% formaldehyde (5 g, 0.05 mol) into the reaction ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistivity | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com