Preparation process of azodicarbonamide
A technology of azodicarbonamide and preparation process, applied in organic chemistry and other directions, can solve problems such as waste of resources, environmental pollution, environmental pollution, etc., and achieve the effect of reducing consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
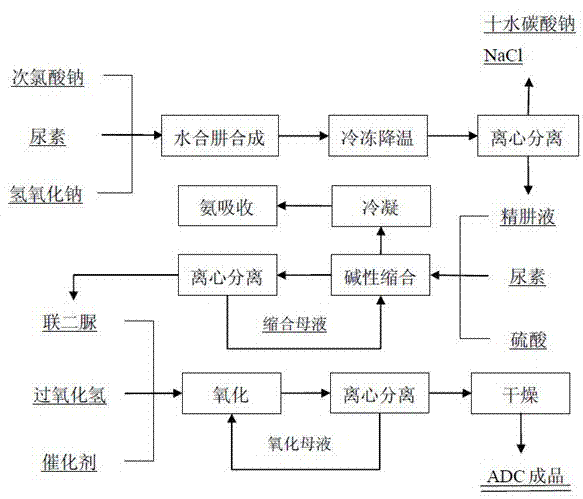
[0055] The preparation process of azodicarbonamide, the process flow see figure 1 ,Proceed as follows:
[0056] 1) Sodium hypochlorite, sodium hydroxide lye with a mass concentration of 11% and a prepared urea solution with a mass concentration of 4% are mixed at a urine-to-chlorine ratio of 1:1 . 189 (mass ratio), chlor-alkali ratio of 1:1.162 (mass ratio) is pumped to the standpipe reactor, and the mass ratio of the amount of catalyst added to the urea used in the reaction is 1 : 83 . Hydrazine hydrate is produced under the action of 33% magnesium sulfate, and the reaction temperature of the pipeline reactor is controlled at 110-114°C to obtain hydrazine hydrate solution (crude product).
[0057] 2) The generated hydrazine hydrate solution is cooled down to -5°C by freezing, and sodium carbonate is precipitated in the form of solid crystallized sodium carbonate decahydrate, which is sent to a centrifuge for solid-liquid separation to remove salts, and the refined hydrazine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com