Method for synthesizing phenyl isothiocyanate
A technology of phenyl isothiocyanate and synthetic method, which is applied in the direction of organic chemistry, can solve the problems of being unsuitable for large-scale industrial production, large environmental impact, long production cycle, etc., and achieves the convenience of industrial production, short reaction cycle, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
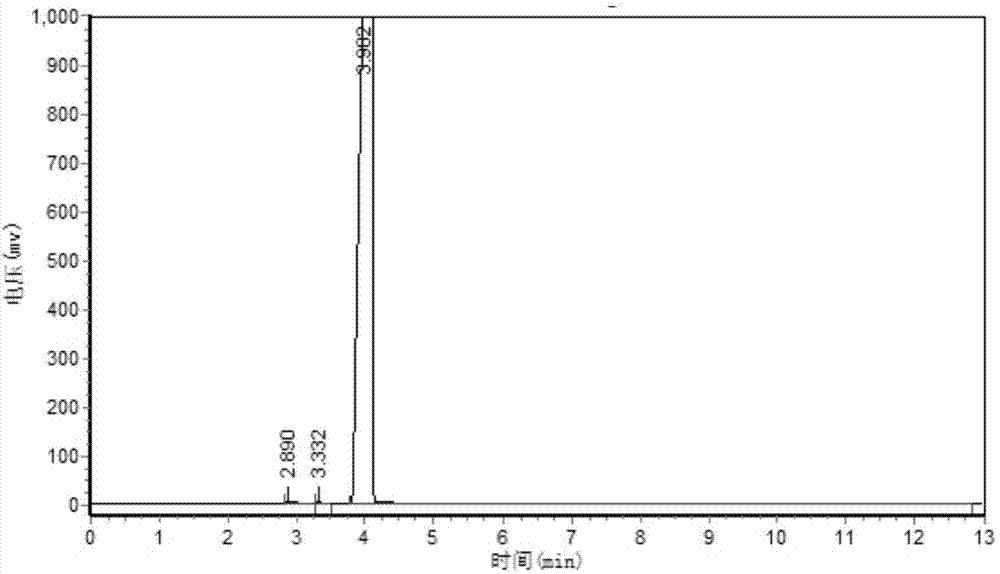
Image
Examples
Embodiment 1
[0036] (1) Add 7.2 kg of deionized water to a reactor, add 1 kg of aniline, and start stirring, then add 2.7 kg of tetramethylthiuram disulfide; heat the reactor to 90°C-100°C and reflux for 4 hours, Stop heating, lower the temperature of the reactor to 70°C-80°C, add 3.3kg of 30% hydrochloric acid, then raise the temperature to 90°C-100°C and reflux for 4 hours, then cool to room temperature.
[0037] (2) Centrifuge to separate the sulfur, wash the sulfur with 0.36kg of petroleum ether, and petroleum ether is used to extract the water phase after separating the oil layer. The acidic aqueous phase was distilled to dryness to obtain dimethylamine hydrochloride. The liquid phase separated by centrifugation was statically separated, the lower oil phase was 1.97 kg of crude phenyl isothiocyanate, the upper aqueous phase was extracted with washed petroleum ether, and the oil phase and petroleum ether solvent were combined.
[0038] (3) The above-mentioned phenyl isothiocyanate cru...
Embodiment 2
[0050] (1) Add 7.2 kg of deionized water to a reactor, add 1 kg of aniline, and start stirring, then add 2.5 kg of tetramethylthiuram disulfide; heat the reactor to 90°C-100°C and reflux for 4 hours, Stop heating, lower the temperature of the reactor to 70°C-80°C, add 3.0kg of 30% hydrochloric acid, then raise the temperature to 90°C-100°C and reflux for 4 hours, then cool to room temperature.
[0051] (2) Centrifuge to separate the sulfur, wash the sulfur with 0.35kg of petroleum ether, and petroleum ether is used to extract the water phase after separating the oil layer. The acidic aqueous phase was distilled to dryness to obtain dimethylamine hydrochloride. The liquid phase separated by centrifugation was allowed to stand for stratification, the lower oil phase was 1.86 kg of crude phenyl isothiocyanate, the upper water phase was extracted with washed petroleum ether, and the oil phase and petroleum ether solvent were combined.
[0052] (3) The crude product of phenyl isot...
Embodiment 3
[0054] (1) Add 7.2 kg of deionized water to a reaction kettle, add 1 kg of aniline, and start stirring, then add 3.0 kg of tetramethylthiuram disulfide; heat the reaction kettle to 90°C-100°C and reflux for 4 hours, Stop heating, lower the temperature of the reactor to 70°C-80°C, add 3.5kg of 30% hydrochloric acid, then raise the temperature to 90°C-100°C and reflux for 4 hours, then cool to room temperature.
[0055] (2) Centrifuge to separate the sulfur, wash the sulfur with 0.40kg of petroleum ether, and petroleum ether is used to extract the water phase after separating the oil layer. The acidic aqueous phase was distilled to dryness to obtain dimethylamine hydrochloride. The liquid phase separated by centrifugation was allowed to stand for stratification, and the lower oil phase was 2.01 kg of crude phenyl isothiocyanate. The upper aqueous phase was extracted with washed petroleum ether, and the oil phase and petroleum ether solvent were combined.
[0056] (3) The crude ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com