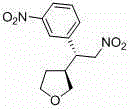
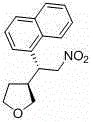
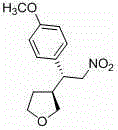
A kind of chiral 3-(2-nitroethyl)tetrahydrofuran compound and its preparation method
A technology of hydroxytetrahydrofuran and nitroethyl is applied in the field of chiral 3-(2-nitroethyl)tetrahydrofuran compound and its preparation, and in the field of tetrahydrofuran compound material, which can solve the problem that the synthesis method of 3-substituted tetrahydrofuran compound is rare, and the hand is limited. The application of 3-substituted tetrahydrofuran compounds is difficult to meet the requirements of practical application, and the effect of good application prospect, novel structure and short reaction time is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 1mmol of 1-nitro, 2-(3-nitrophenyl)-ethene, 2.5mmol of 2-hydroxytetrahydrofuran and 0.4mmol of catalyst (R 2 = phenyl, R 3 = trimethylsilyl group) was dissolved in 3 mL of chloroform, 1 mmol of acetic acid was added, and the reaction was carried out at 25 °C for 12 hours until the reaction of nitrostyrene was complete. The reaction solution was washed with 5 ml of 1 mol / L hydrochloric acid, then extracted with dichloromethane, the combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Add 12mL of dichloromethane to the residue, then add 3mmol of triethylsilane, slowly add 3mmol of boron trifluoride ether under stirring at room temperature, and react at room temperature for 2 hours; then the solvent is removed by rotary evaporation, and the residue is subjected to silica gel column chromatography. Ether: ethyl acetate = 2:1 elution, the light yellow solid is obtained as the pr...
Embodiment 2
[0042] Put 1mmol of 1-nitro, 2-(3-nitrophenyl)-ethene, 2.5mmol of 2-hydroxytetrahydrofuran and 0.4mmol of catalyst (R 2 =3,5-ditrifluoromethylphenyl, R 3 = trimethylsilyl) was dissolved in 3mL toluene, 0.4mmol acetic acid was added, and the reaction was carried out at 25°C for 15 hours until the nitrostyrene reaction was complete. The reaction solution was washed with 5 ml of 1 mol / L hydrochloric acid, then extracted with dichloromethane, the combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Add 12mL of dichloromethane to the residue, then add 3mmol of triethylsilane, slowly add 3mmol of boron trifluoride diethyl ether under stirring at room temperature, and react at room temperature for 2 hours; then the solvent is removed by rotary evaporation, and the residue is subjected to silica gel column chromatography. Ether: ethyl acetate = 2:1 elution, 170 mg of light yellow solid produc...
Embodiment 3
[0044] Put 1mmol of 1-nitro, 2-(3-nitrophenyl)-ethene, 2.5mmol of 2-hydroxytetrahydrofuran and 0.4mmol of catalyst (R 2 = phenyl, R 3 = tert-butyldimethylsilyl) was dissolved in 3 mL of N,N-dimethylformamide, 0.4 mmol acetic acid was added, and the reaction was carried out at 25 °C for 12 hours until the nitrostyrene reaction was complete. The reaction solution was washed with 5 ml of 1 mol / L hydrochloric acid, then extracted with dichloromethane, the combined organic layers were washed with saturated brine, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Add 12mL of dichloromethane to the residue, then add 3mmol of triethylsilane, slowly add 3mmol of boron trifluoride diethyl ether under stirring at room temperature, and react at room temperature for 2 hours; then the solvent is removed by rotary evaporation, and the residue is subjected to silica gel column chromatography. Ether: ethyl acetate = 2:1 eluted to obtain 181 mg of light ye...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com