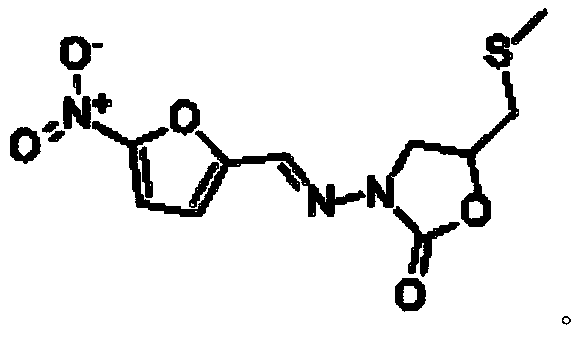
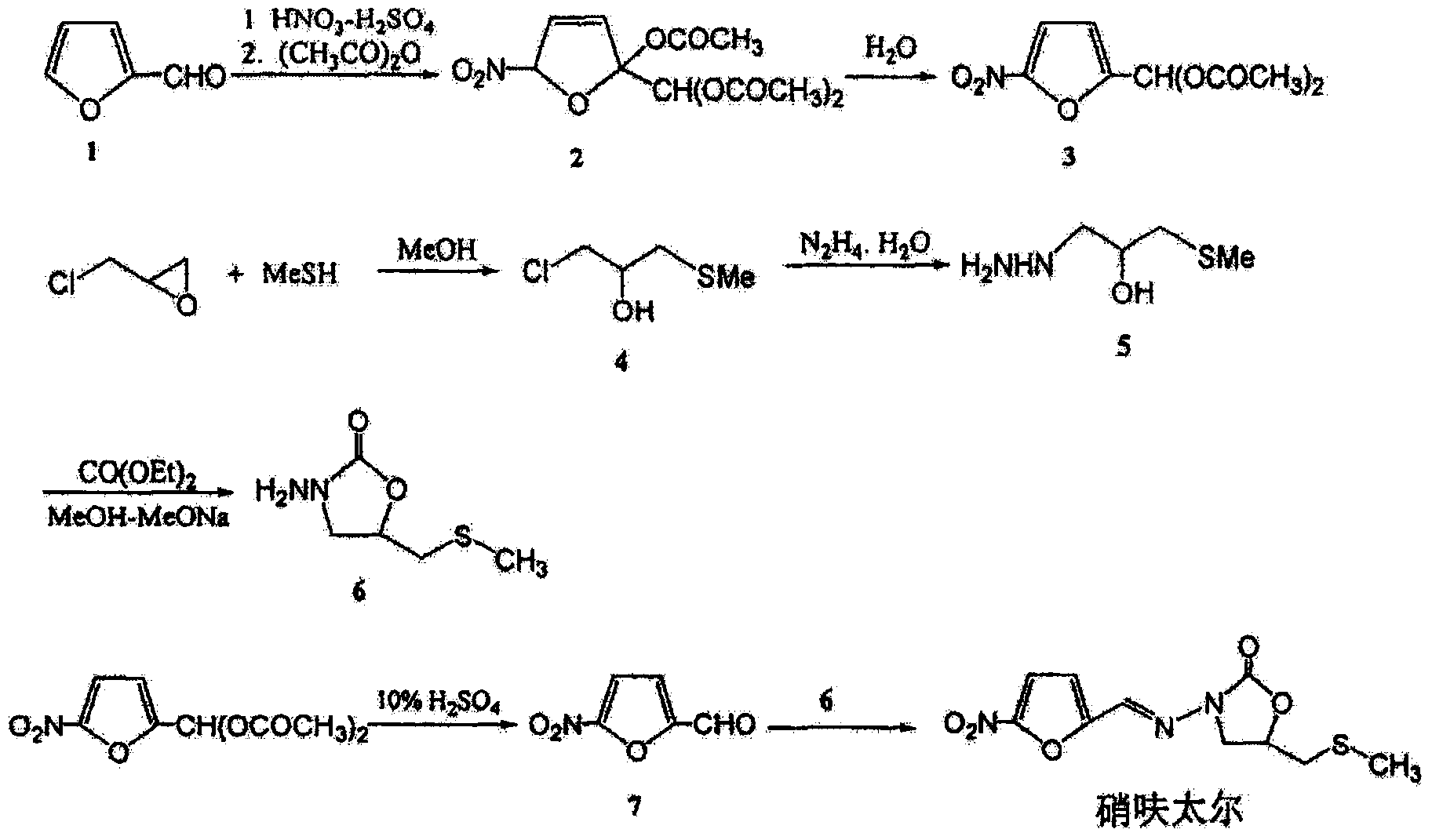
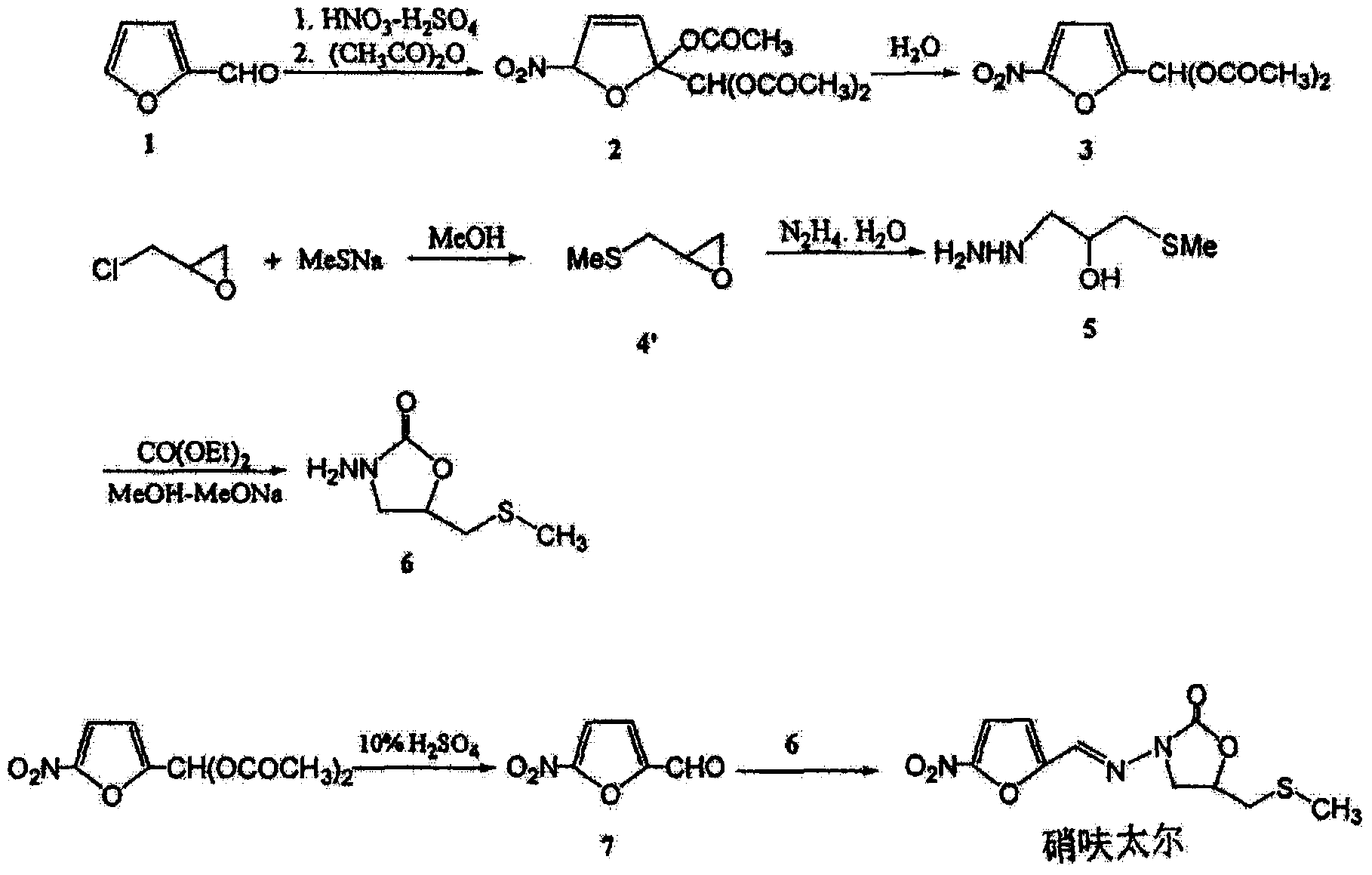
Synthetic method of nifuratel
A synthesis method and nifuratel technology are applied in the field of pharmaceutical synthesis and can solve the problems of low purity, many product impurities, low reaction rate and yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Carry out the preparation of nifuratel according to the following process:
[0044] (1) Put 1mol epichlorohydrin, 280ml dichloromethane, and 0.05mol tetrabutylammonium bromide into the reaction flask, and slowly add 20% aqueous solution of sodium methylmercaptide (sodium methylmercaptan 6.5mol) dropwise under stirring , the dropping time is 2 hours, and the temperature is controlled at 15-25°C during the dropping process; after the dropping is completed, the temperature is kept at 18°C for 3 hours. Epoxypropyl methyl sulfide, yield is 78%;
[0045] (2) Put hydrazine hydrate into another reaction bottle according to the molar ratio of epoxypropyl methyl sulfide and hydrazine hydrate as 1:1.05, stir and raise the temperature to 95°C, slowly drop the epoxy obtained in step (1) Propyl methyl sulfide, dropwise time is 1h; then react at 95-98°C for 3h, after the reaction is completed, lower to room temperature; first concentrate the reaction solution to viscous, then disti...
Embodiment 2
[0050] Carry out the preparation of nifuratel according to the following process:
[0051] (1) Put 1mol of epichlorohydrin, 280ml of dichloromethane, and 0.055mol of tetrabutylammonium bromide into the reaction flask, and slowly add 20% aqueous solution of sodium methyl mercaptide (7 mol of sodium methyl mercaptide) dropwise under stirring. The dropping time is 2 hours, and the temperature is controlled at 15-25°C during the dropping process; after the dropping, keep the temperature at 20°C for 3 hours, after the reaction, distill the reaction solution under reduced pressure, and collect the fractions of bp2050-55°C to obtain purified ring Oxypropyl methyl sulfide, yield is 80%;
[0052] (2) According to the molar ratio of glycidyl methyl sulfide and hydrazine hydrate as 1:1.1, put hydrazine hydrate into another reaction bottle, stir and raise the temperature to 95°C, slowly drop the epoxy obtained in step (1) Propyl methyl sulfide, dropwise time is 1h; then react at 95-98°C ...
Embodiment 3
[0057] Carry out the preparation of nifuratel according to the following process:
[0058] (1) Put 1mol of epichlorohydrin, 280ml of dichloromethane, and 0.06mol of tetrabutylammonium bromide into the reaction flask, and slowly add 20% aqueous solution of sodium methyl mercaptide (8 mol of sodium methyl mercaptide) dropwise under stirring. The dropping time is 2 hours, and the temperature is controlled at 15-25°C during the dropping process; after the dropping, keep the temperature at 20°C for 3 hours, after the reaction, distill the reaction solution under reduced pressure, and collect the fractions of bp2050-55°C to obtain purified ring Oxypropyl methyl sulfide, yield is 82%;
[0059] (2) According to the molar ratio of glycidyl methyl sulfide and hydrazine hydrate as 1:1.2, put hydrazine hydrate into another reaction bottle, stir and raise the temperature to 95°C, slowly drop the epoxy obtained in step (1) Propyl methyl sulfide, dropwise time is 1h; then react at 95-98°C f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com