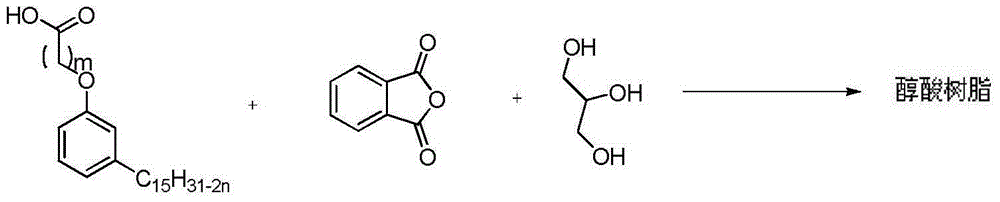
A kind of preparation method of alkyd resin based on cardanol
A technology of alkyd resin and cardanol, applied in the chemical field, can solve the problems of insufficient strength and high cost of alkyd resin, achieve the advantages of performance and environmental friendliness, reduce the cost of raw materials, and reduce the cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
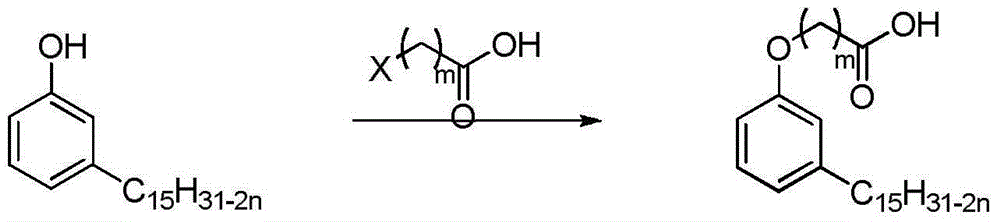
[0031] Cardanol and chloroacetic acid are first used as raw materials to synthesize cardanol ether ethyl acid, and its specific synthesis is as follows: cardanol 195g (0.65mol) is added in a four-necked flask, solvent 95% ethanol is added, and sodium hydroxide (48g , 1.2mol) of 50% aqueous solution was slowly dropped into the system, the reaction was stirred at room temperature for 30min, then heated to reflux for 30min, then chloroacetic acid (122g, 1.3mol) was added dropwise to the system, and the reaction was continued to be heated to reflux for 60min , and then vacuum evaporated the excess ethanol in the system, then added hydrochloric acid (1M) to adjust the pH>2, added 300mL ethyl acetate to separate layers, washed the organic phase with distilled water three times, then dried with anhydrous sodium sulfate, filtered, filtered The cake was rinsed twice with ethyl acetate (60 mL), the obtained organic phases were combined and the solvent was evaporated to obtain a pale yell...
Embodiment 2
[0038] First, cardanol and 3-chloropropionic acid were used as raw materials to synthesize cardanol ether ethyl acid. The specific synthesis was as follows: 195 g (0.65 mol) of cardanol was added to a four-necked flask, and 95% ethanol was added as a solvent, and then the hydrogen oxidized A 50% aqueous solution of sodium (48g, 1.2mol) was slowly dripped into the system, the reaction was stirred at room temperature for 30min, then heated to reflux for 30min, then 141.5g3-chloropropionic acid (1.3mol) was added dropwise to the system, the The reaction was continued to heat and reflux for 60 minutes, then the excess ethanol in the system was evaporated in vacuum, then hydrochloric acid (1M) was added to adjust the pH>2, 300mL ethyl acetate was added to separate layers, the organic phase was washed three times with distilled water, and then washed with anhydrous sodium sulfate After drying and filtering, the filter cake was rinsed twice with ethyl acetate (60 mL), the obtained org...
Embodiment 3
[0046] First, cardanol and 4-chlorobutyric acid were used as raw materials to synthesize cardanol butyl acid ether. The specific synthesis is as follows: 195 g (0.65 mol) of cardanol was added to a four-necked flask, and 95% ethanol was added as a solvent, and then the hydrogen was oxidized A 50% aqueous solution of sodium (48g, 1.2mol) was slowly dripped into the system, the reaction was stirred at room temperature for 30min, then heated to reflux for 30min, then 159.315g of chlorobutyric acid (1.3mol) was added dropwise to the system, the reaction Continue to heat and reflux for 60 minutes, then vacuum evaporate excess ethanol in the system, then add hydrochloric acid (1M) to adjust the pH>2, add 300mL ethyl acetate to separate layers, wash the organic phase with distilled water three times, and then dry it with anhydrous sodium sulfate , filtered, and the filter cake was rinsed twice with ethyl acetate (60 mL), the resulting organic phases were combined and the solvent was e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com