A pharmaceutical composition for preventing and treating osteoporosis and its preparation method
A technology for osteoporosis and composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations containing active ingredients, etc., can solve the problems of low quality control standards, low content of active ingredients, and single effect, and achieve the goal of raw material medicine The effect of wide distribution of resources, clear active ingredients, and low toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1, the preparation of Epimedium and Ligustrum lucidum extract and the preparation of pharmaceutical composition
[0053] 1. Prepare Epimedium and Ligustrum lucidum extracts by decocting:
[0054] (1) Preparation of Epimedium extract: Take 8000g of Epimedium, chop it, and use 90% ethanol aqueous solution as the extraction solvent to extract 3 times, the first time is 1g Epimedium: 15mL extraction solvent The extraction was carried out at a ratio of 70°C for 3 hours; the second and third times were extracted by mixing the medicinal residues obtained in the previous time with the extraction solvent (the ratio was 1g medicinal residues: 10mL extraction solvent) for extraction at 70°C for 2 hours. Combine the extracts, remove the ethanol in the extract, then extract the extract with petroleum ether for 3 times (to remove chlorophyll), discard the petroleum ether phase, and collect the water phase; D-101 macroporous adsorption resin on the water phase, wash with wa...
Embodiment 2
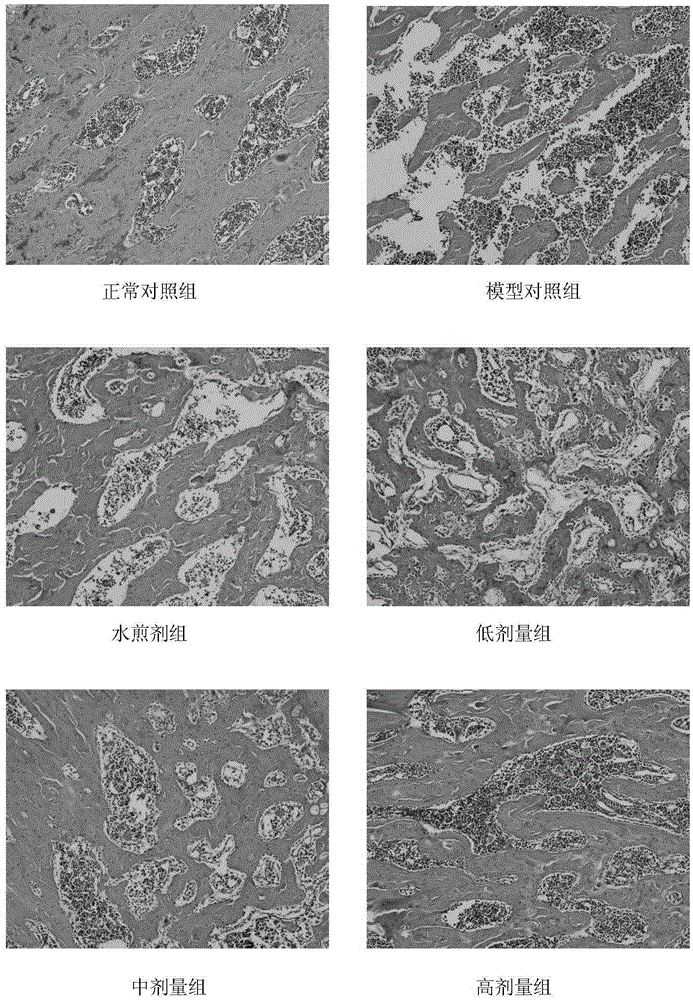
[0058] Example 2, the effect of the active ingredient extract of Epimedium Ligustrum lucidum on retinoic acid-induced osteoporosis in rats
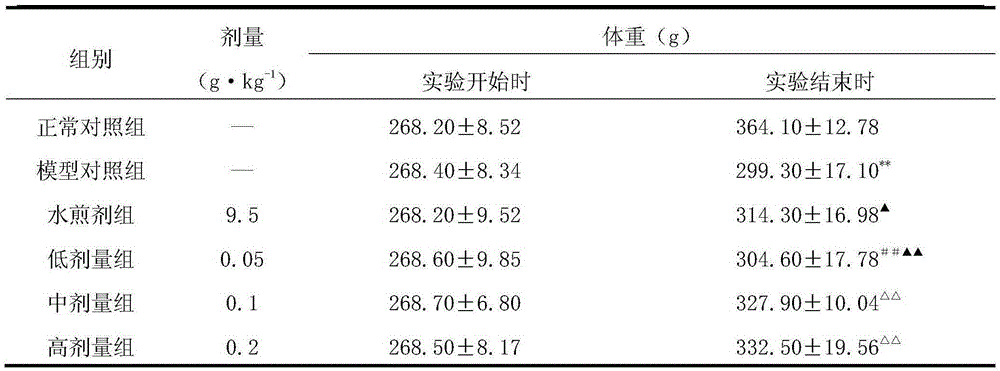
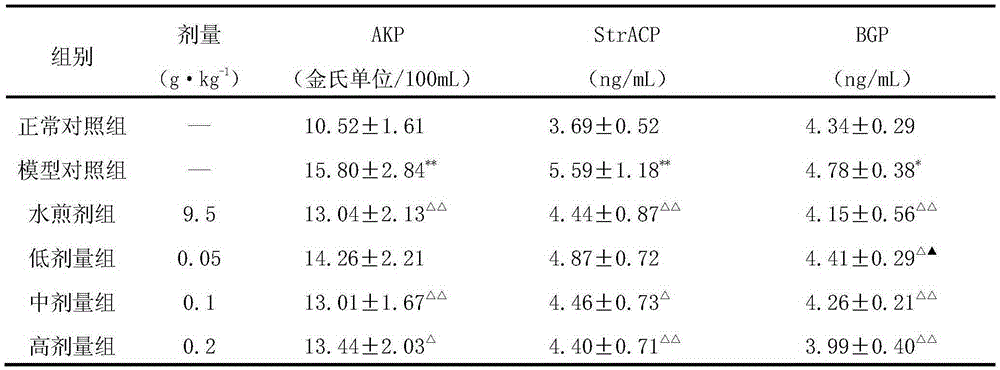
[0059] 1. Sixty 3-month-old male Wistar rats were randomly divided into 6 groups, namely, the normal control group, the model control group, the epimedium privet decoction control group (abbreviation: water decoction group), epimedium Low, medium and high dose groups of the extract of Huo Ligumi (respectively referred to as low, medium and high dose groups), 10 rats in each group.
[0060] Except for the normal control group, rats in other groups were treated with retinoic acid suspension (preparation method: first prepare 0.5% (0.5g / 100mL) carboxymethylcellulose sodium (CMC-Na) solution with distilled water, and then prepare 7mg·mL -1 retinoic acid suspension) by 10mL·kg -1 The normal control group was given intragastric administration with an equal volume of 0.5% CMC-Na, and the model was established continuously for 2 weeks. At the ...
experiment example 3
[0111] Experimental example 3, the effect of the active ingredient extract of Epimedium Ligustrum lucidum on the rat model of glucocorticoid-induced osteoporosis
[0112] Clean-grade healthy SD rats, half male and half male, weighing (200±20) g, were divided into 6 groups according to the random number table method, the same as in Experimental Example 2. Except for the normal control group, rats in other groups were given alternate intramuscular injections of dexamethasone 1 mg·kg into the inner thighs. -1 Body weight, 2 times a week, a total of 8 weeks, normal control group rats were given an equal volume of normal saline. At the same time of modeling, the animals in each group were intragastrically administered with corresponding drugs or normal saline, once a day, for a total of 8 weeks. Dosage, execution, sampling, and statistical methods for experimental results are the same as in Experimental Example 2.
[0113] 1. Comparison of bone mineral density in each group of ra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com