Synthetic method of 2-imine-1,3-oxathiole compound
A technology of heterocyclopentene and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of long reaction time, high reaction temperature, many side reactions, etc., and achieve the effects of high reaction efficiency, mild reaction conditions, and low experimental cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The synthesis of 2-benzimine-1,3-oxathiobenzocyclopentene comprises the following steps:
[0070] ①Put 50mmol 2-iodophenol, 50mmol sodium bicarbonate, 1mmol copper nitrate trihydrate, 100mmol phenyl isothiocyanate, 5mmol N,N,N'N'-tetramethylethylenediamine, 200mL water into a 500mL beaker. , magnetically stirred at 20°C, and reacted for 2 hours;
[0071] ② After the reaction, extract 4 times with ethyl acetate and saturated brine, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain a crude product;
[0072] ③ The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 20:1) to obtain a white solid, namely 2-benzimine-1,3-oxathiobenzene Pentalene, yield 91%, melting point: 73-74°C.
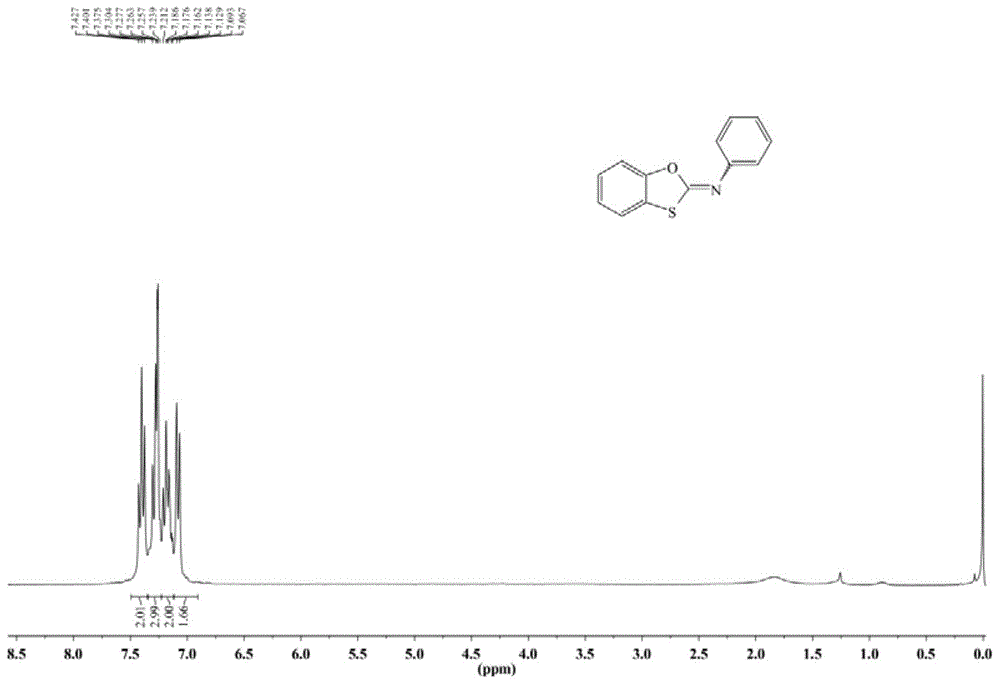
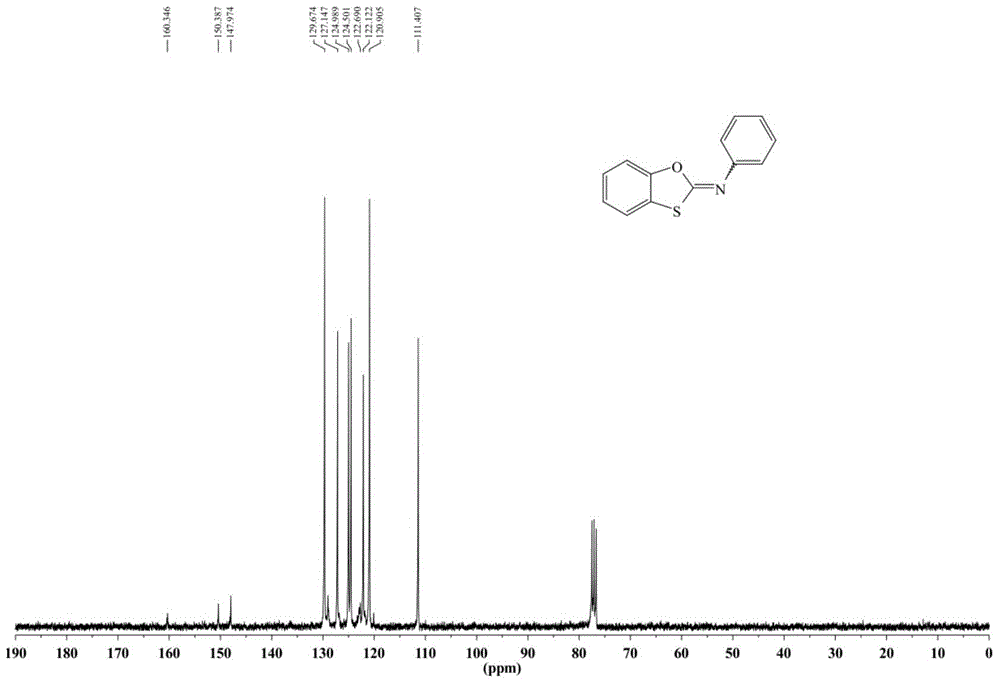
[0073] 1 HNMR (300MHz, CDCl 3 ):δ=7.40(t,J=7.8Hz,2H),7.30-7.24(m,3H),7.21-7.13(m,...
Embodiment 2
[0076] The synthesis of 2-(4-trifluoromethylphenyl)imine-1,3-oxathiobenzocyclopentene comprises the following steps:
[0077] ①In a 500mL beaker, add 50mmol 2-iodophenol, 50mmol sodium carbonate, 60mmol 4-trifluoromethylphenylisothiocyanate, 0.03mmol copper nitrate trihydrate, 5mmol N,N,N'N'-tetramethylethane Diamine, 135mL water, magnetic stirring at 40°C, react for 4 hours;
[0078] ② After the reaction, extract 5 times with ethyl acetate and saturated brine, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain a crude product;
[0079] ③ The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 40:1) to obtain a white solid, namely 2-(4-trifluoromethylphenyl)imine- 1,3-Oxathiabenzocyclopentene, yield 86%, melting point: 121-122°C.
[0080] 1 HNMR (300MHz, CDCl 3 ):δ...
Embodiment 3
[0084] The synthesis of 2-(4-chlorophenyl)imine-1,3-oxathiobenzocyclopentene comprises the following steps:
[0085] ①In a 500mL beaker, add 45mmol 2-iodophenol, 40mmol sodium hydroxide, 80mmol 4-chlorophenylisothiocyanate, 0.5mmol copper chloride dihydrate, 8mmol N,N,N'N'-tetramethylethylenedioxide Amine, 150mL water, magnetic stirring at 30°C, react for 1 hour;
[0086] ② After the reaction, extract 5 times with ethyl acetate and saturated brine, combine the organic layers, dry over anhydrous sodium sulfate, and concentrate under reduced pressure with a rotary evaporator to remove the solvent in the solution to obtain a crude product;
[0087] ③ The crude product was separated and purified by 300-400 mesh silica gel column chromatography (volume ratio of petroleum ether: ethyl acetate = 20:1) to obtain a white solid, namely 2-(4-chlorophenyl)imine-1,3 - Oxathiabenzocyclopentene, yield 89%, melting point: 80-81°C.
[0088] 1 HNMR (300MHz, CDCl 3 ):δ=7.34(d,J=8.7Hz,2H),7.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com