Catalyst for preparation of synthetic gas through partial oxidation of methane, and preparation method and application thereof
A methane catalytic part and catalyst technology, applied in the field of methane partial oxidation to synthesis gas catalyst and its preparation and application, can solve the problems of catalyst performance, loss of metal active components, catalyst structure change, etc. Large-scale production, high methane conversion rate, and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
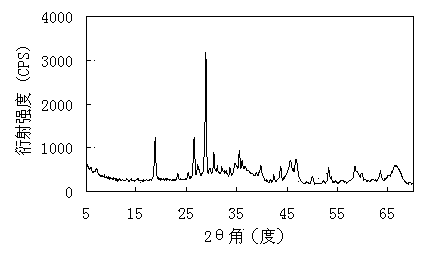
Image
Examples
Embodiment 1
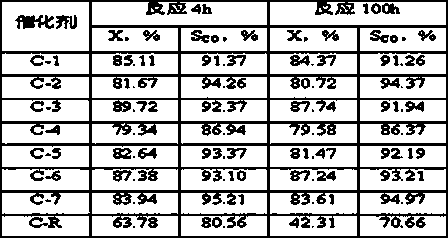
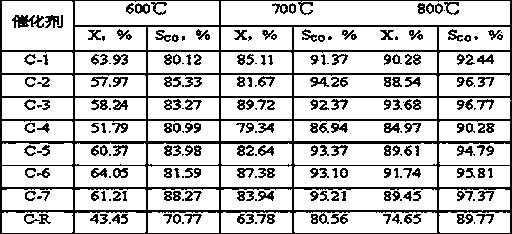
[0020] Weigh 2.91gNi(NO 3 ) 2 6H 2 O, 2.91gCo(NO 3 ) 2 6H 2 O, dissolved in 30g deionized water to prepare an aqueous solution containing nickel nitrate and cobalt nitrate. 1.16g NH 4 VO 3 Dissolve in 20g deionized water, then use with NH 4 VO 3 Treat with equimolar oxalic acid to obtain a dark blue homogeneous solution containing vanadium precursor, and mix it evenly with the solution containing nickel nitrate and cobalt nitrate, add 10.0 g of alumina carrier ground to more than 200 mesh, and heat it in a water bath to 70 ℃. Under the condition of stirring, slowly add 8wt% ammonia solution dropwise to the above solution until the pH value of the solution is about 6, and nitric acid can be used for callback. After stirring at a constant temperature of 70°C for 6 hours, stop the stirring, continue to keep the temperature constant until there is no clear water, dry at 110°C for 24 hours, and air roast at 700°C for 8 hours to shape the catalyst sample. Ni in the final ...
Embodiment 2
[0023] Weigh 2.91gNi(NO 3 ) 2 6H 2 O, 1.46gCo(NO 3 ) 2 6H 2 O, dissolved in 30g deionized water to prepare an aqueous solution containing nickel nitrate and cobalt nitrate. 0.35g NH 4 VO 3 Dissolve in 20g deionized water, then use with NH 4 VO 3 Treat with equimolar oxalic acid to prepare a dark blue homogeneous solution containing vanadium precursor, and mix it evenly with the solution containing nickel nitrate and cobalt nitrate, add 6.0g of alumina carrier ground to 200 mesh or more, and heat in a water bath to 60 ℃. Slowly add 10wt% ammonia solution dropwise to the above solution under agitation until the pH value of the solution is about 8, and nitric acid can be used for callback. After stirring at a constant temperature of 60°C for 10 hours, stop the stirring, continue to hold the temperature until there is no clear water, dry at 120°C for 16 hours, and calcinate in air at 800°C for 4 hours to shape the catalyst sample. Ni in the final catalyst 1 co 0.5 V ...
Embodiment 3
[0026] Weigh 8.72gNi(NO 3 ) 2 6H 2 O, dissolved in 30g deionized water to prepare an aqueous solution containing nickel nitrate. 1.75g NH 4 VO 3 Dissolve in 20g deionized water, then use with NH 4 VO 3 Treat with equimolar oxalic acid to prepare a dark blue homogeneous solution containing vanadium precursor, and mix it evenly with the solution containing nickel nitrate, add 9.0 g of alumina carrier ground to 200 mesh or more, and heat to 80°C in a water bath. Slowly add 15wt% ammonia solution dropwise to the above solution under stirring condition until the pH value of the solution is about 10, which can be adjusted back with nitric acid. After stirring at a constant temperature of 80°C for 6 hours, stop the stirring, continue to keep the temperature constant until there is no clear water, dry at 80°C for 14 hours, and air roast at 700°C for 8 hours to shape the catalyst sample. Ni in the final catalyst 1 V 0.5 o 2.75 The weight content is 30%, recorded as C-3.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com