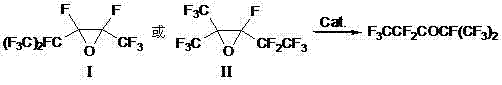
Preparation process of perfluoro-2-methyl-3-pentanone
A preparation technology, methyl technology, applied in the field of preparation technology of perfluoro-2-methyl-3-pentanone, to achieve the effect of high efficiency of reaction equipment, easy operation and low equipment requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1: Synthesis of perfluoro-2-methyl-2,3-epoxypentane by continuous liquid phase epoxidation
[0038] Follow these steps:
[0039] a. Prefabricate 164.7kg of newly prepared sodium hypochlorite solution with an available chlorine content of 16.98%, add 19.7L of acetonitrile, mix well to make a sodium hypochlorite-acetonitrile solution, and weigh 98.6kg (0.329kmol) of 97.4% perfluoro-2- Methyl-2-pentene, the perfluoro-2-methyl-2-pentene of the present invention is obtained by telomerization and isomerization of hexafluoropropylene by a known method;
[0040] b. After measuring the sodium hypochlorite-acetonitrile solution and perfluoro-2-methyl-2-pentene according to the ratio with a metering pump, cool them to about -10°C through the second heat exchanger and the first heat exchanger respectively After that, it is continuously sent to the mixer for mixing;
[0041] c. The mixed reaction materials in the mixer are continuously sent into a pipeline-type liquid phas...
Embodiment 2
[0047] The continuous liquid phase epoxidation method synthesizes perfluoro-2-methyl-2,3-epoxypentane according to the following steps:
[0048] The method operation identical with embodiment 1, difference is that sodium hypochlorite quality is 163.3kg, and available chlorine content is 17.11%, and acetonitrile consumption is 19.7L, and perfluoro-2-methyl-2-pentene consumption is 98.6kg (0.329 kmol) content is 98.21%, and the reaction temperature is 0°C.
[0049] Results: The quality of the crude epoxide was 99.2kg (0.314kmol), the content was 97.83%, and the yield was 95.14%.
Embodiment 3
[0051] The continuous liquid phase epoxidation method synthesizes perfluoro-2-methyl-2,3-epoxypentane according to the following steps:
[0052] The same method operation as in Example 1, the difference is that the quality of sodium hypochlorite is 164.6kg, the available chlorine content is 18.03%, the amount of diethylene glycol dimethyl ether is 22.6L, perfluoro-2-methyl-2-pentene The dosage is 113.3kg (0.329kmol) and the content is 98.21%.
[0053] Results: The quality of the crude epoxide was 114.2kg (0.356kmol), the content was 97.62%, and the yield was 95.12%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com