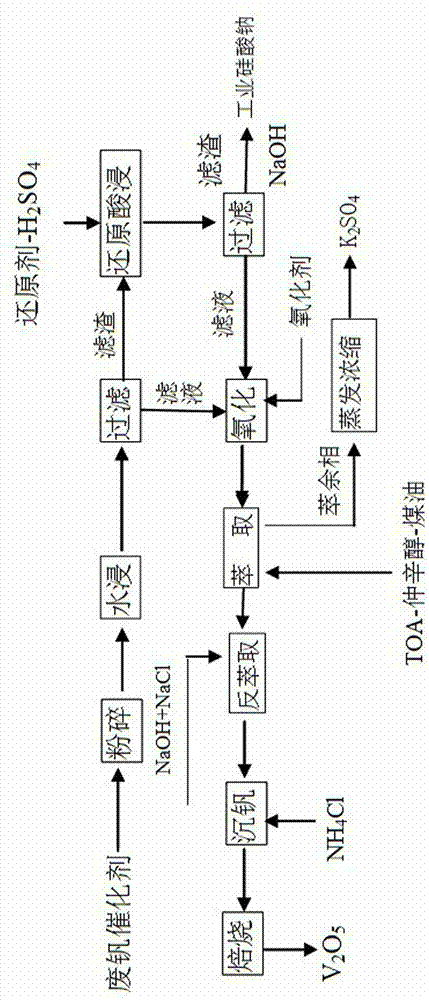
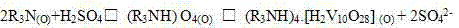Method for recovering vanadium, potassium and silicon from waste vanadium catalyst
A waste vanadium catalyst and recovery method technology, applied in the direction of silicate, alkali metal silicate, process efficiency improvement, etc., can solve the problems of recovery rate to be further improved, long process flow, waste of resources, etc., to achieve significant economic Benefits and social benefits, high purity, high value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Present embodiment 1 carries out as follows:
[0068] 1. Add 200 g of water to 100 g of spent vanadium catalyst with a particle size of 300 μm, and leach for 2 hours at a temperature of 90 ° C. After the water immersion, filter to obtain the water leaching filtrate and water leaching filter residue, and wash the water leaching filter residue with clear water until neutral. Separately collect water leaching residue, water leaching filtrate and lotion;
[0069] 2. In 350g mass ratio of 11% sulfuric acid solution, add 3.1 g of potassium sulfite reducing agent, and divide it into 4 parts, add the first part of leaching solution to the water leaching residue obtained in step 1, at 100 React at ℃ for 2 hours, let it stand for precipitation, and absorb the supernatant to obtain the reducing acid immersion solution. Repeat the same operation 3 more times. After reducing acid leaching, filter the reducing acid leaching filtrate and filter residue, wash the reducing acid leachi...
Embodiment 2
[0078] Present embodiment 2 carries out as follows:
[0079] 1. Add 150g of water to 100g of spent vanadium catalyst with a particle size of 355μm, leaching for 2.5 hours at a temperature of 85°C, filter the water leaching filtrate and filter residue after water leaching, wash the water leaching filter residue until neutral, and collect Water leaching residue, water leaching filtrate, lotion;
[0080] 2. In 250g of sulfuric acid solution with a mass ratio of 11%, add 2.4g of sodium sulfite reducing agent and divide it into 4 parts. Add the first leaching solution to the water leaching residue obtained in step 1, react at 90°C for 3 hours, let it stand for precipitation, and absorb the supernatant to obtain the reducing acid leaching solution. Repeat the same operation 3 more times. After reducing acid leaching, filter the reducing acid leaching filtrate and filter residue, wash the reducing acid leaching filter residue with water until neutral, collect the reducing acid leac...
Embodiment 3
[0089] Present embodiment 3 carries out as follows:
[0090] 1. Add 250g of water to 100g of spent vanadium catalyst with a particle size of 250μm, and leaching at 95°C for 1.5 hours. After the water immersion, filter to obtain the water leaching filtrate and filter residue. The water leaching filter residue is washed with water until neutral, and collected separately. Water leaching residue, water leaching filtrate, lotion;
[0091] 2. In 400g of 11% sulfuric acid solution, add 3.6 g of potassium sulfite, and divide it into 4 parts, add the first part of leaching solution to the water leaching residue obtained in step 1, and react at 95 ° C for 2.5 Hours, let it stand for precipitation, and absorb the supernatant to obtain the reducing acid immersion solution. Repeat the same operation 3 more times. After reducing acid leaching, filter the reducing acid leaching filtrate and filter residue, wash the reducing acid leaching filter residue with water until neutral, collect th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
| Granularity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com