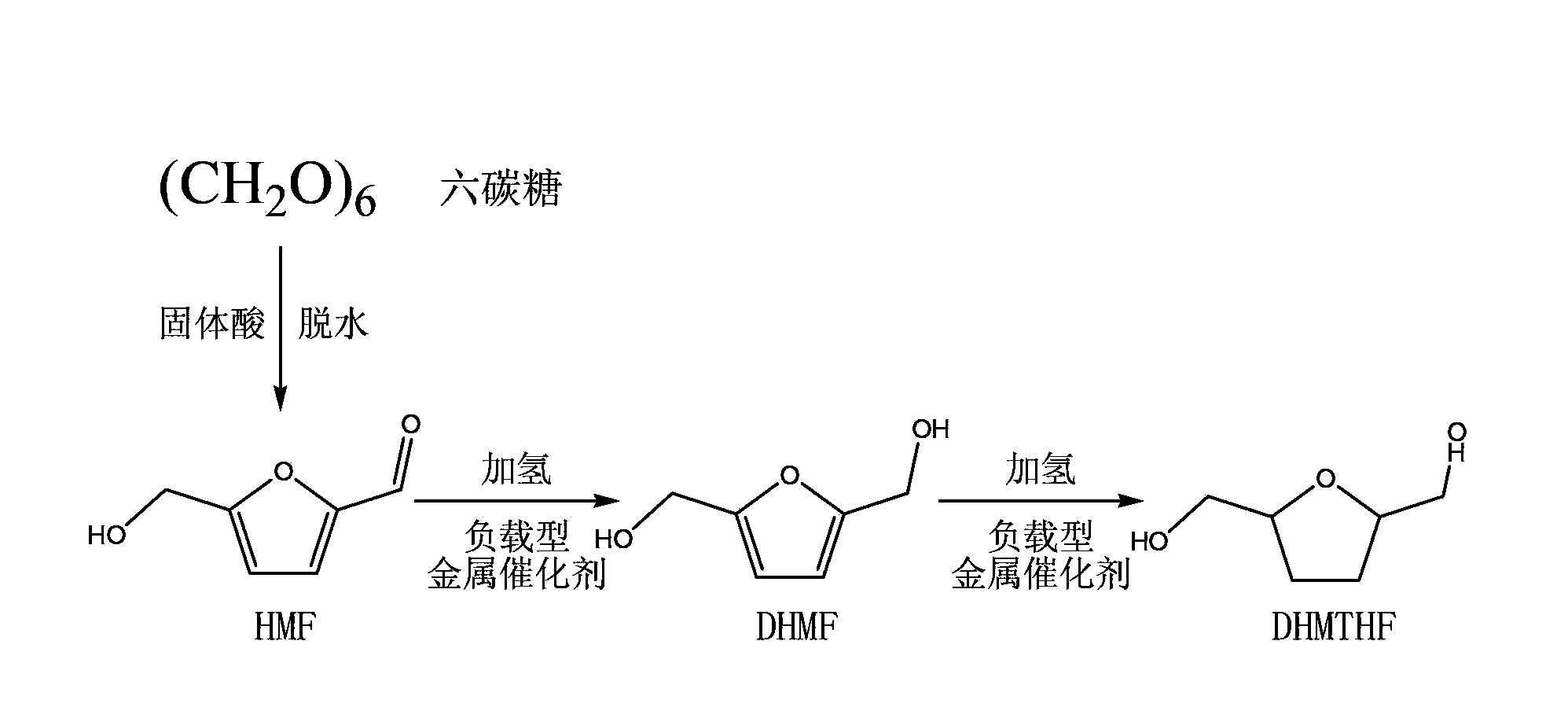
2, 5-dihydroxy methyl furan or 2, 5-dihydroxy methyl tetrahydrofuran synthesis method
A technology of dimethyloltetrahydrofuran and dimethylolfuran, which is applied in the direction of organic chemistry, can solve the problems of high pollution, high energy consumption, and high cost, and achieve the effects of wide sources, high catalytic activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Fructose dehydration prepares HMF
[0025] Dissolve 1.32g fructose in 11.85g isopropanol, add 0.132g Amberlyst-15, heat to 100°C, stir rapidly, and react for 4 hours. Afterwards, the reaction kettle was cooled with ice water to room temperature, the reaction solution was centrifuged, a certain amount of supernatant was taken in a 10mL volumetric flask, and the ethanol was fixed to volume. The concentration of HMF was quantitatively analyzed by gas chromatography, and the yield of HMF reached 65%.
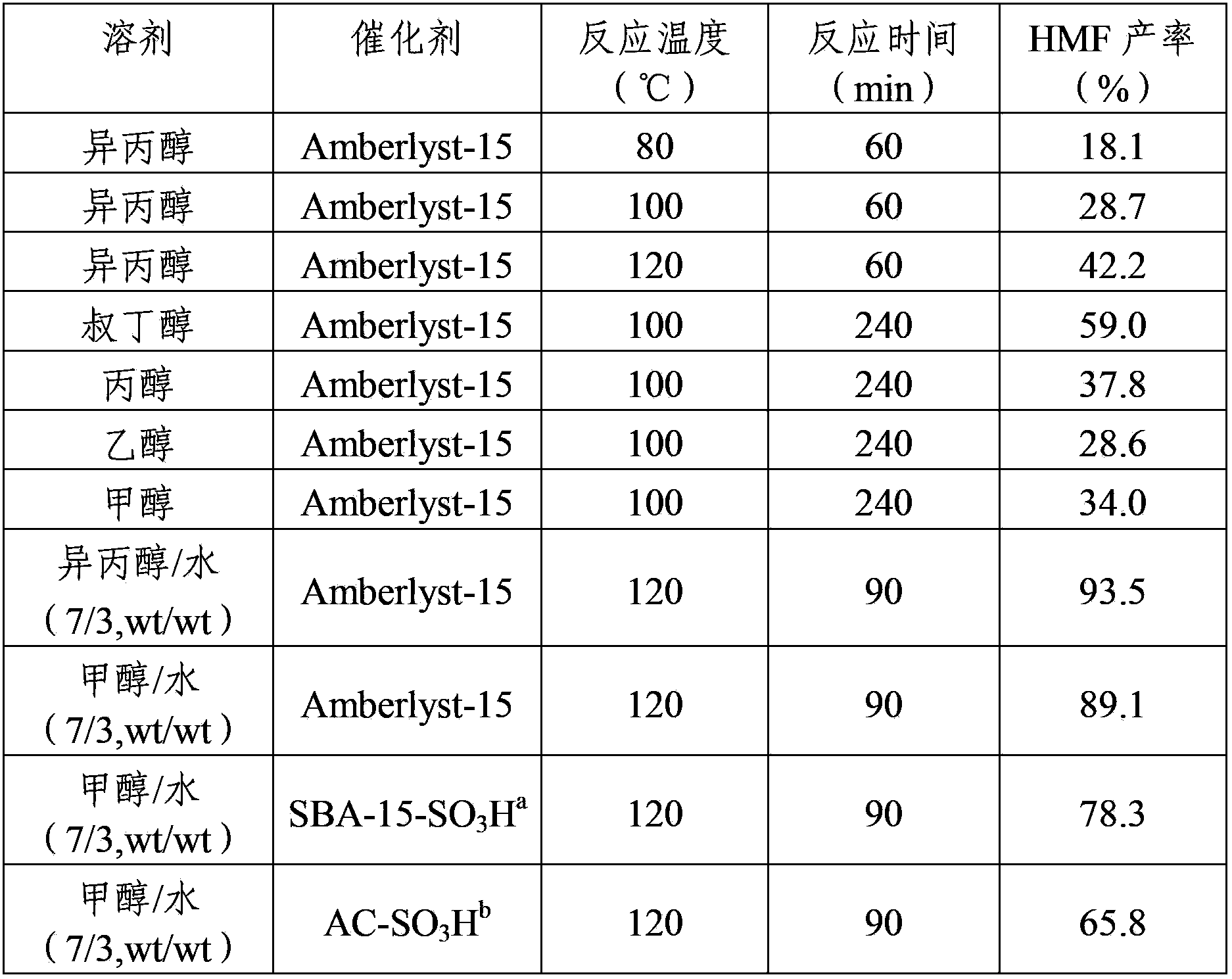
[0026] Table 1 shows the reaction results of HMF prepared by dehydration of fructose in different reaction temperatures, times and solvent systems.
[0027] Table 1 Preparation of HMF by dehydration of fructose
[0028]
[0029] a SBA-15-SO 3 H is the sulfonic acid functionalized SBA-15 synthesized by one-step method.
[0030] b AC-SO 3 H is a sulfonated activated carbon material.
Embodiment 2
[0031] Embodiment 2HMF hydrogenation prepares 2,5-dimethylolfuran or 2,5-dimethyloltetrahydrofuran
[0032] This example studies the preparation of hydrogenation catalysts, including the influence of different main catalysts, auxiliary agents, and supports, and its catalytic performance for the conversion of HMF at room temperature.
[0033]The hydrogenation catalyst was prepared by equal volume impregnation method. Add the dried carrier to the metal salt solution (the amount of the metal salt is determined by the metal content of the active component), stir evenly, soak at room temperature for 24 hours, and dry for 12 hours after the metal ions are adsorbed on the carrier. After the prepared catalyst was reduced in a hydrogen atmosphere at 350 °C, it was used to catalyze the conversion of HMF.
[0034] HMF hydrogenation conversion: add 10mL 1.25wt% HMF solution and 0.1g reduced catalyst into a 50mL autoclave, seal and replace the air 5 times. Charge hydrogen gas at a pressu...
Embodiment 3
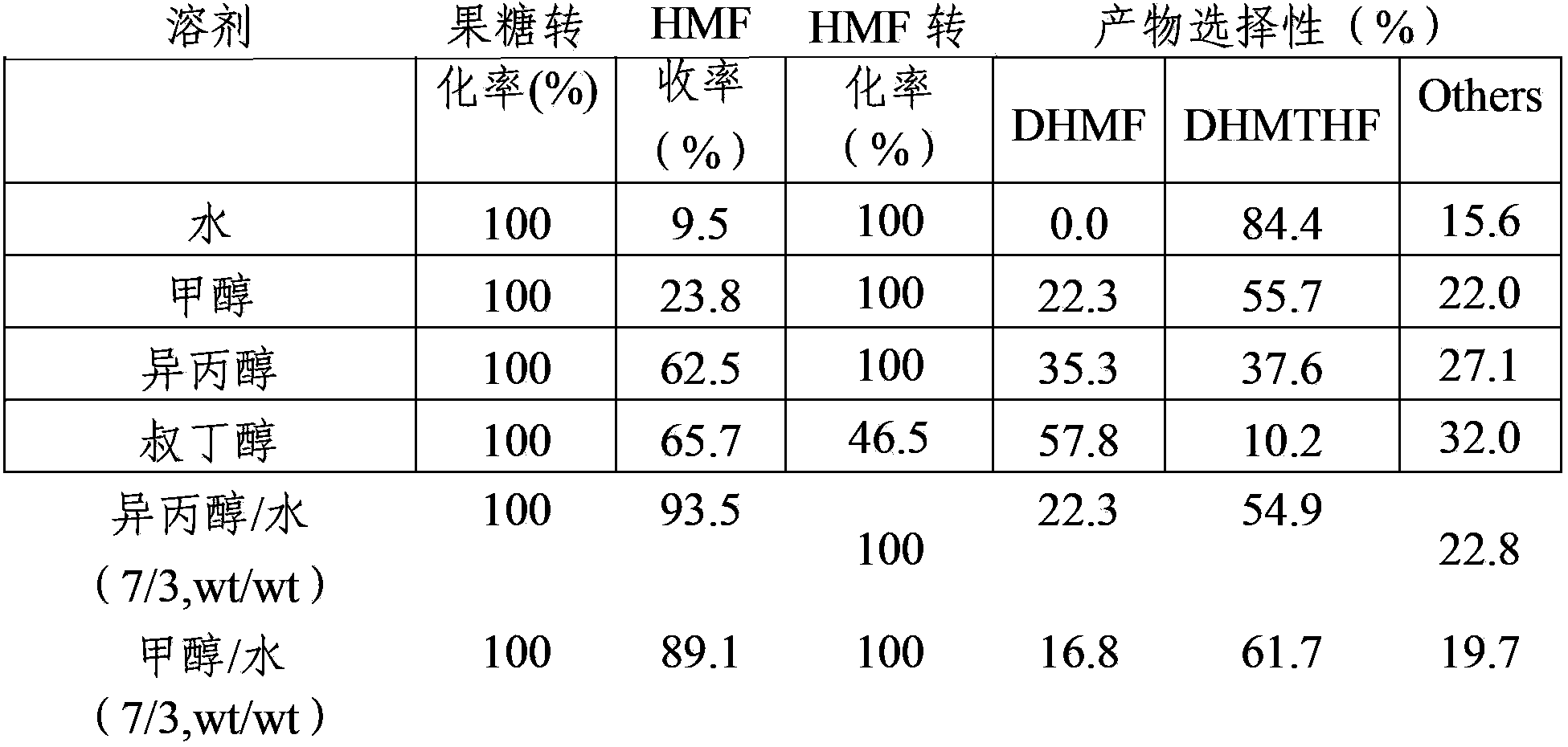
[0040] The influence of embodiment 3 solvent on HMF hydrogenation reaction
[0041] The influence of different solvent systems on the hydrogenation performance of HMF at room temperature has been studied. The catalyst is No. 1 in Example 2. Other reaction processes are the same as in Example 2. The reaction results are shown in Table 3. When water is used as a solvent, HMF has the highest hydrogenation activity, 2 , The yield of 5-dimethyloltetrahydrofuran (DHMTHF) was 83.0%.
[0042] The solvent effect of table 3 HMF hydrogenation reaction
[0043]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com