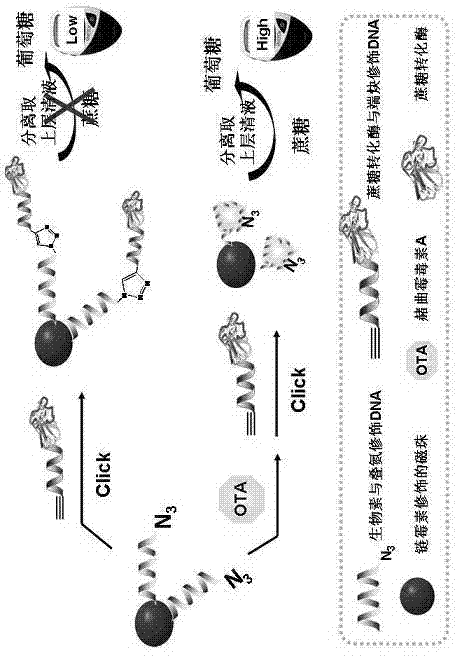
Method for portably and rapidly detecting ochratoxin A
An ochratoxin, rapid technique for use in analytical chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
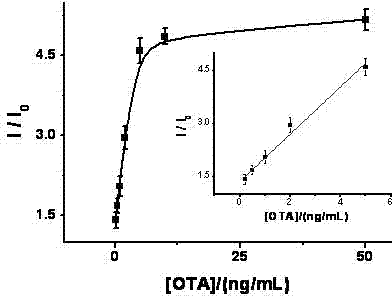
[0032] A portable method for rapidly detecting ochratoxin A, the specific steps are as follows:
[0033]1. Prepare sucrose invertase-terminal alkyne-modified DNA: First, mix 30 μL of 0.3 mM thiol-terminal alkyne-modified DNA (thiol-terminal alkyne-modified DNA sequence from 5' to 3' end is: 5'-alkynyl- AAA AAA AAA AAA- thiol-3'), 2.0 μL 30 mM tris-(2-formylethyl)phosphine (TCEP) and 2.0 μL 1.0 M PBS buffer solution (a phosphate buffer solution prepared by sodium monohydrogen phosphate and sodium dihydrogen phosphate, pH is 5.5), mixed evenly, and incubated at room temperature for 1.0 hour to activate thiol-terminal alkyne modified DNA. After the end, an ultrafiltration centrifuge tube with a molecular weight of 10K was used to separate and wash 5 times. Next add 1.0 mg of sulfo-4-(N-maleimidomethyl)cyclohexane-1-carboxylate succinimidyl ester sodium salt (Sulfo-SMCC) to 400 μL of 20 mg / mL sucrose conversion In the enzyme solution, mix well and shake at room temperature for ...
Embodiment 2
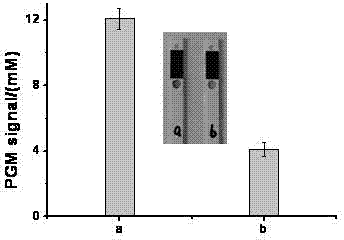
[0039] The specificity of a portable rapid detection method for ochratoxin A, the specific steps are as follows:
[0040] Under the same experimental conditions as in Example 1, four other toxins were used instead of ochratoxin A to measure the blood glucose meter response values of four other toxins respectively, and compared with the response values of ochratoxin A, then The specificity of the method of the present invention was investigated. The four toxins are aflatoxin B 1 (AFB 1 ), aflatoxin M 1 (AFM 1 ), zearalenone (F-2), both at a concentration of 5.0 ng / mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com