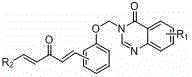
Pentadienone compound containing quinazolinone aryloxy group, preparation method and application
A technology containing quinazolinone aryloxy group and pentadienone is applied in the field of pentadienone derivatives and their preparation, and can solve the problem that no pentadienone derivatives have ever been synthesized and have less resistance to plant viruses. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1, (1E,4E)-3-((2-(3-oxo-5-phenyl-1,4-pentadien-1-yl)phenoxy)methyl)quinazoline- Synthesis of 4-(3H)-ketone (A1)
[0066] (1) Synthesis of quinazolin-4-(3H)-one
[0067]Add 27.5g (0.2mol) of anthranilic acid and 36.1g (0.8mol) of formamide into a 250mL three-necked flask equipped with a thermometer and a condenser to keep the temperature at 135-150°C. During this process, the system turns from light gray to turbid The liquid turns into a yellow-brown clear liquid, TLC followed the reaction, and the reaction was complete in about 5 hours. Slowly add 100mL of water to the system after cooling down to 100°C naturally to decompose excess formamide, and a light gray solid precipitates out at the same time. After natural cooling Transfer to a large beaker, then add a large amount of water and there is still off-white solid precipitation, suction filtration, drying to obtain 28.88g of the product, recrystallization with absolute ethanol to obtain 27.5g of brown-white n...
Embodiment 2
[0078] Embodiment 2, the synthesis of compound (A2)
[0079] Steps (1)-(4) are the same as the steps (1)-(4) of Embodiment 1;
[0080] The difference between step (5) and step (5) of Example 1 is that: add 0.78g (5.56mmol) p-chlorobenzaldehyde instead of 0.58g (5.5mmol) benzaldehyde, react for 10h, and obtain 1.23g yellow solid, melting point ° C, yield Rate 86.5%;
[0081] The difference between step (6) and Example 1 step (6) is: add 0.37g (1.31mmol) 1-(4-chlorophenyl)-5-(2-hydroxyphenyl)-1,4-pentadiene -3-one, replace 0.33g (1.31mmol) 1-(2-hydroxyphenyl)-1,4-pentadien-3-one, react for 8h, recrystallize to get 0.23g white solid, melting point 195-197℃ , yield 39.6%.
Embodiment 3
[0082] Embodiment 3, the synthesis of compound (A3)
[0083] Step (1)-(4) is the same as embodiment 1(1)-(4);
[0084] Step (5) is the same as embodiment 1 (5) method and condition. The difference is that 0.68g (5.49mmol) 1-(2-fluorophenyl)-5-(2-hydroxyphenyl)-1,4-pentadien-3-one was added and reacted for 10h to obtain 1.10g yellow solid, Yield 82.1%.
[0085] Step (6) is the same as embodiment 1 (6) method and condition. The difference is that 0.35g (1.31mmol) 1-(2-fluorophenyl)-5-(2-hydroxyphenyl)-1,4-pentadien-3-one was added, reacted for 5h, and recrystallized to obtain 0.25g yellow Solid, melting point 142-144°C, yield 44.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com