Test method for pulse-activating preparation adopting pilose asiabell root
A technology of Shengmai and preparations, which is applied in the content determination of Shengmai preparations (Dangshenfang) and the content determination of traditional Chinese medicine compound preparations. Improve the level of quality evaluation and quality control, save testing time and experimental costs, the effect of large workload and inspection costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Optimization of chromatographic conditions (taking Shengmaiyin (Dangshen Fang) as an example)
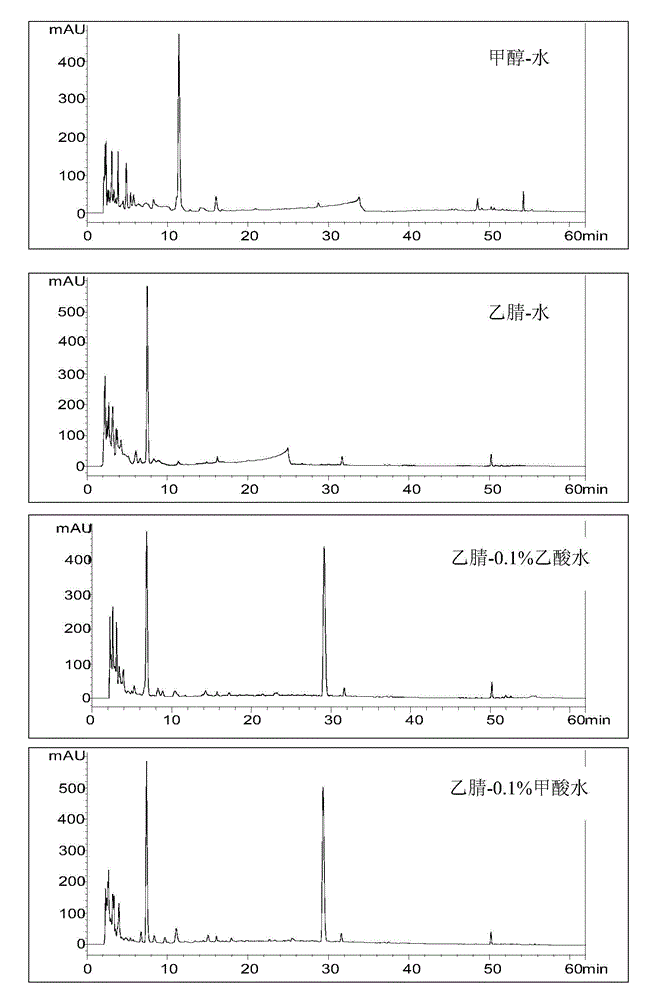
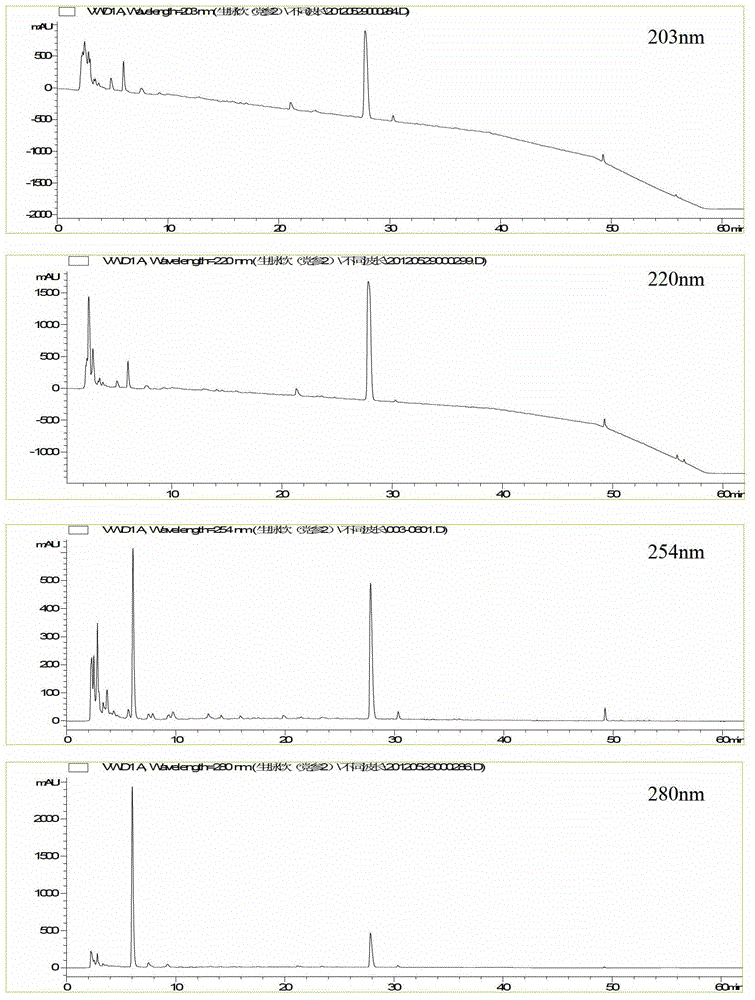
[0045] 1. Selection of detection wavelength
[0046] The liquid chromatograms ( figure 1 ), the preparation method of the test solution is: accurately pipette 920 μL of Shengmaiyin (Dangshen Fang) sample, add 80 μL of acetonitrile, centrifuge at high speed for 10 minutes (speed 13000 rpm), take the supernatant, filter it with a 0.45 μm microporous membrane, That is, the test solution was obtained. The chromatographic conditions are: chromatographic column: Agilent Extend C18 column (4.6mm×250mm, 5μm); mobile phase: A is acetonitrile, B is 0.1% formic acid aqueous solution, and the gradient elution is set according to the elution program in the table below; flow rate: 1.0mL / min; column temperature: 25°C; injection volume: 10μL.
[0047] Table 1: Elution program table
[0048]
[0049] Table 1 shows: at the beginning, the volume percentage concentration of mobi...
Embodiment 2
[0055] The selection of embodiment 2 need testing solution preparation conditions
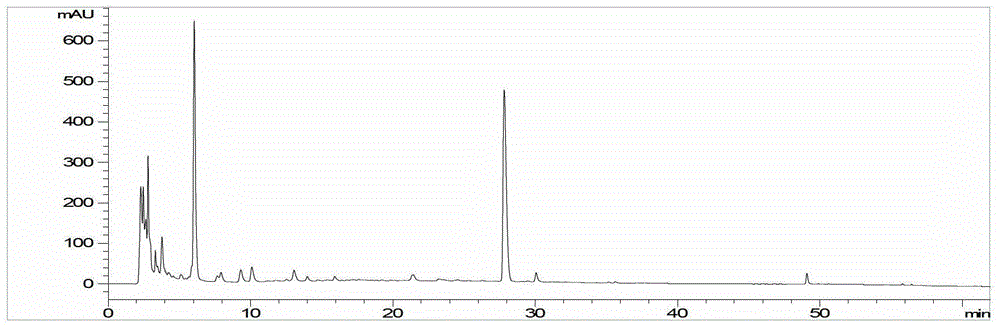
[0056] ①Preparation condition 1 of the test solution: Precisely pipette 920 μL of Shengmaiyin (Dangshen Fang) sample, add 80 μL of acetonitrile, centrifuge at a high speed for 10 minutes (speed 13000 rpm), take the supernatant, and filter it with a 0.45 μm microporous membrane to obtain the Test solution ①, detected by high performance liquid chromatography, chromatographic conditions: chromatographic column: Agilent Extend C18 column (4.6mm×250mm, 5μm); mobile phase: A is acetonitrile, B is 0.1% formic acid aqueous solution, and gradient elution is carried out , the elution schedule is the same as Table 1; flow rate: 1.0mL / min; column temperature: 25°C; detection wavelength: 254nm; injection volume: 10μL. The resulting chromatograms are shown in image 3 .
[0057] ②Preparation condition 2 of the test solution: Accurately measure 10mL of Shengmaiyin (Dangshen Fang) sample, extract 3 times wi...
Embodiment 3
[0062] Example 3 Separation of samples under different gradient elution procedures (taking Shengmaiyin (Dangshen Fang) test solution as an example)
[0063] The preparation method of the test solution is: accurately pipette 920 μL of Shengmaiyin (Dangshen Fang) sample, add 80 μL of acetonitrile, centrifuge at a high speed for 10 minutes (speed 13000 rpm), take the supernatant, and filter it with a 0.45 μm microporous membrane to obtain The test solution.
[0064] 1. Elution procedure 1, see chromatogram Figure 7 .
[0065]
[0066] 2. Elution procedure 2, see chromatogram Figure 8 .
[0067]
[0068] 3. Elution procedure 3, see chromatogram Figure 9 .
[0069]
[0070] 4. Elution procedure 4, see chromatogram Figure 10 .
[0071]
[0072] The above samples were all eluted on an Agilent Extend C18 column (4.6mm×250mm, 5μm), flow rate: 1.0mL / min; column temperature: 25°C; detection wavelength: 254nm; injection volume: 10μL.
[0073] Under the above elutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com