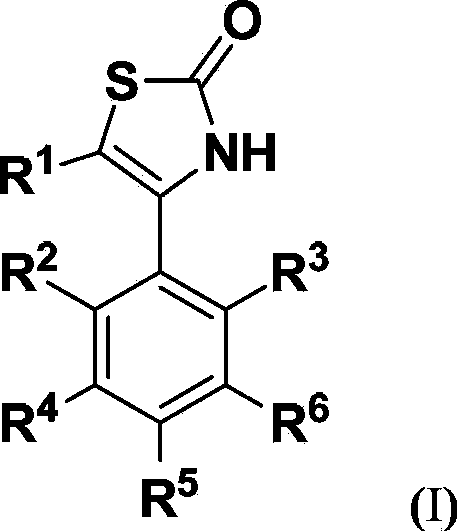
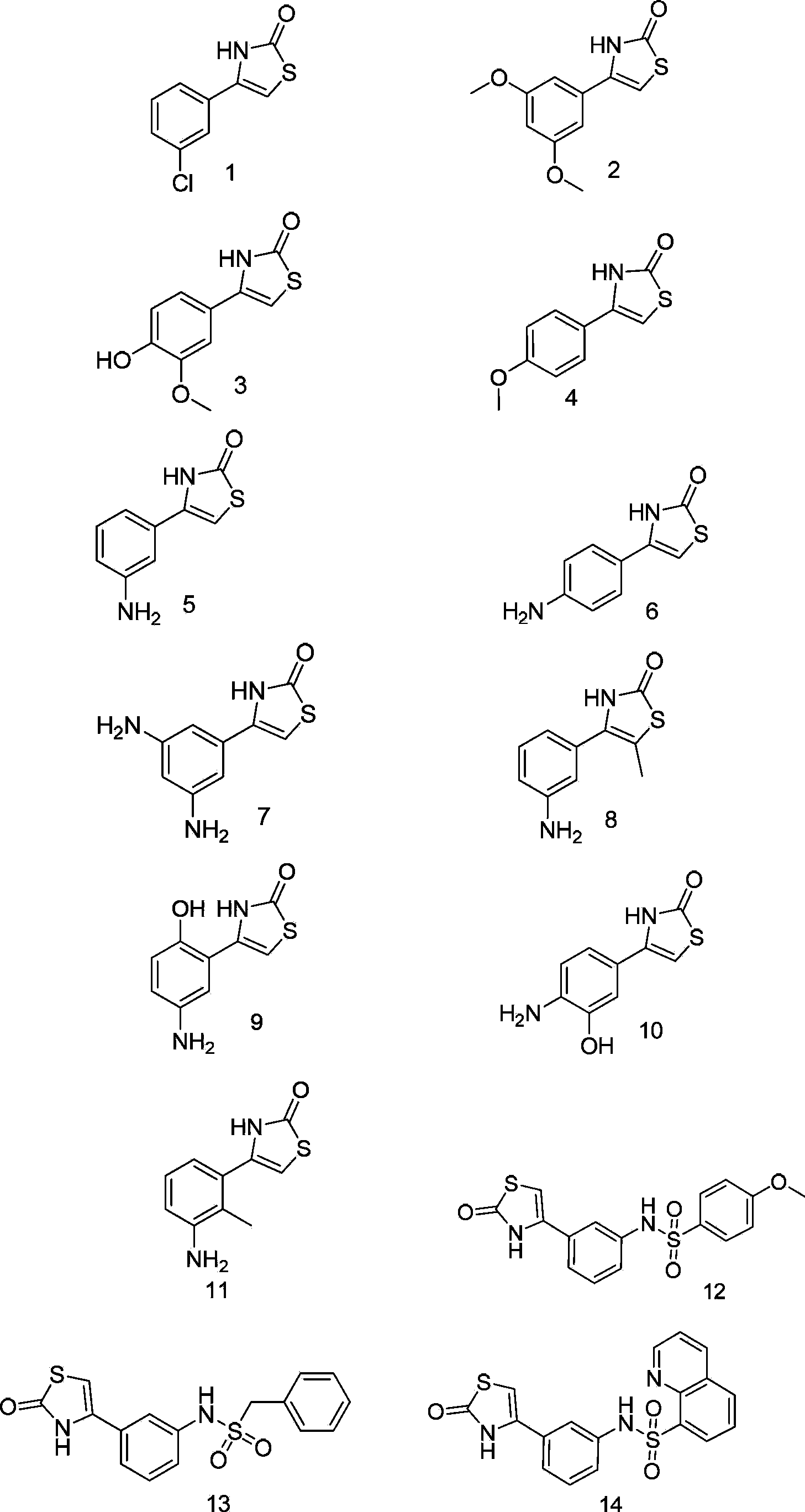
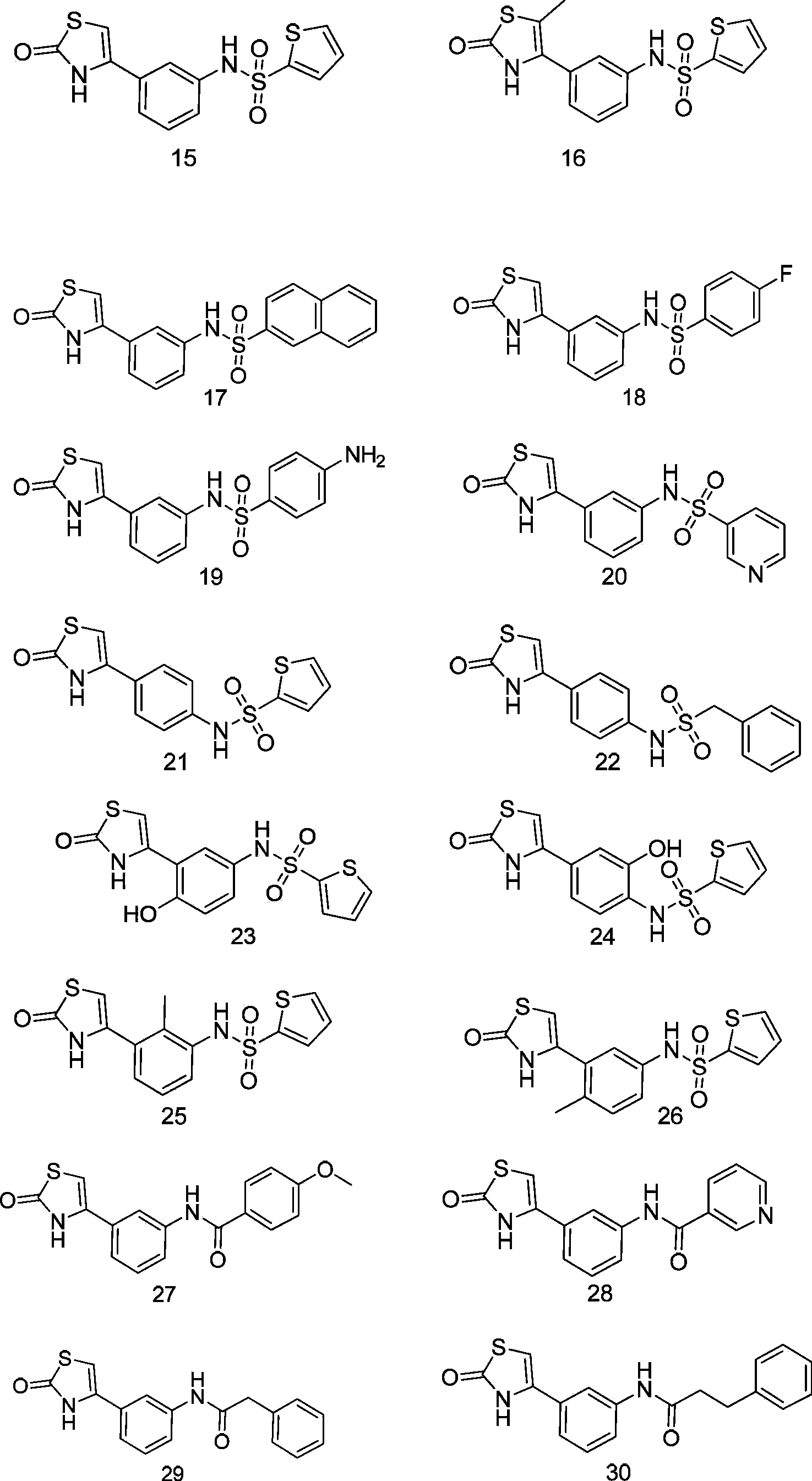
Thiazoline compounds and pharmaceutical composition and application thereof
A technology of dihydrothiazolone and compounds, which is applied in the field of drug synthesis and can solve problems such as interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073]
[0074] The synthetic route is:
[0075]
[0076] Reagents and conditions: a) copper bromide, ethyl acetate, 80°C; b) potassium thiocyanate, acetone, room temperature; 50% sulfuric acid, glacial acetic acid, 100°C
[0077] a) 3-Chloroacetophenone (0.2g, 1.294mmol) and copper bromide (0.347g, 1.552mmol) were successively dissolved in ethyl acetate (30mL), heated to 80°C and stirred overnight, and the reaction solution was cooled to room temperature, Add 30 mL of ethyl acetate, extract with 100 mL of water and 100 mL of saturated sodium chloride solution successively, separate the ethyl acetate layer, dry with anhydrous sodium sulfate, evaporate the organic solvent under reduced pressure, and purify the residue by flash silica gel column chromatography. Elute with petroleum ether / ethyl acetate (V / V=10:1) to obtain 220 mg of 2-bromo-1-(3-chlorophenyl)ethanone as a white solid with a yield of 72.8%. 1 H NMR (400MHz, CDCl3) δ7.94 (td, J=5.8, 3.0Hz, 1H), 7.88–7.83(m, ...
Embodiment 5
[0082]
[0083] The synthetic route is:
[0084]
[0085]Steps a and b were carried out in the same manner as in Preparation Example 1. The specific steps of step c are: 4-(3-nitrophenyl)thiazol-2(3H)-one (1.05g, 4.73mmol) was dissolved in 20mL ethanol, ammonium chloride (1.264g, 23.66mmol) was dissolved in Add 5mL of water to the reaction solution, then add reduced iron powder (1.319g, 23.66mmol), heat the reaction solution to 80°C and stir for 30 minutes, dilute the reaction solution with ethyl acetate, filter through diatomaceous earth, and concentrate the filtrate under reduced pressure to leave The product was purified by flash silica gel column chromatography, eluting with dichloromethane / methanol (V / V=20:1), to obtain 700 mg of the title compound as a pale yellow solid, with a yield of 77%. MS(ES):m / z 193.1[M+H] + ; 1 H NMR(400MHz,DMSO)δ11.62(s,1H),7.05(t,J=7.7Hz,1H),6.78–6.73(m,2H),6.57(ddd,J=8.0,2.2,1.0Hz, 1H),6.53(d,J=1.6Hz,1H),5.21(s,2H).
[0086] The fol...
Embodiment 9
[0089]
[0090] The synthetic route is:
[0091]
[0092] The specific steps of step a are: dissolve 2-hydroxyacetophenone (11.3g, 83mmol) in 100mL glacial acetic acid, add fuming nitric acid (5.57mL, 125mmol) dropwise within 30 minutes under ice bath, and continue under ice bath Stir for 1 hour, warm to room temperature and stir for 16 hours. The reaction solution was poured into ice water, the precipitated solid was filtered, washed with water, and dried. The solid was purified by flash silica gel column chromatography and eluted with petroleum ether / ethyl acetate (V / V=5:1) to obtain 1-(2-hydroxyl -5.3 g of 5-nitrophenyl) ethyl ketone, which is a pale yellow solid, with a yield of 35.2%. 1 H NMR (400MHz, DMSO) δ6.65(t, J=5.3Hz, 2H), 6.51(s, 1H), 6.47(dd, J=8.5, 2.7Hz, 1H), 4.56(s, 2H).
[0093] Steps b, c and d are carried out in the same manner as steps a, b and c of Preparation Example 5 to obtain the title compound of Example 9, 1 H NMR (400MHz, DMSO) δ6.65(t, J=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com