Ionizable cation lipid compound and application thereof
A technology of cationic lipids and compounds, applied in the field of gene therapy, can solve problems such as low efficiency, and achieve the effect of reducing cytotoxicity, easy degradation and effective delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1. Ionizable cationic lipid L1
[0048] Lysine, linoleic acid and oleyl alcohol were selected as raw materials, and L1 lipid was synthesized by Shanghai Maofu Chemical Technology Co., Ltd. according to the relevant technology; all of which were purified by HPLC and identified by mass spectrometry, the purity was greater than 95%, and the molecular weight was consistent with the theoretical value;
[0049] Effect verification:
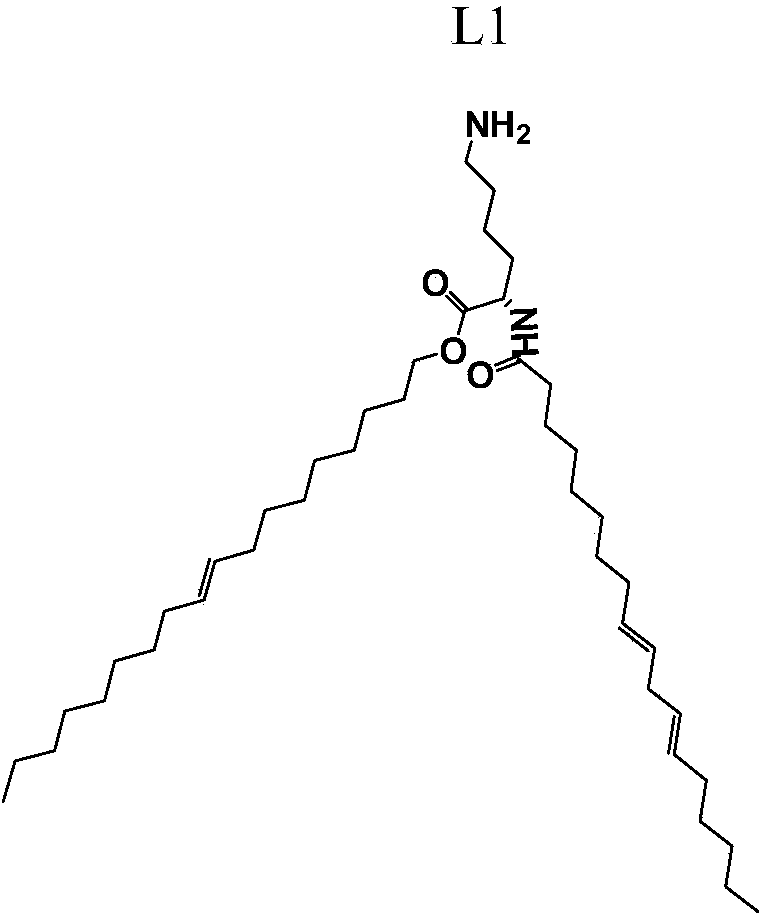
[0050] (1) figure 1 is the structural diagram of the ionizable cationic lipid L1;
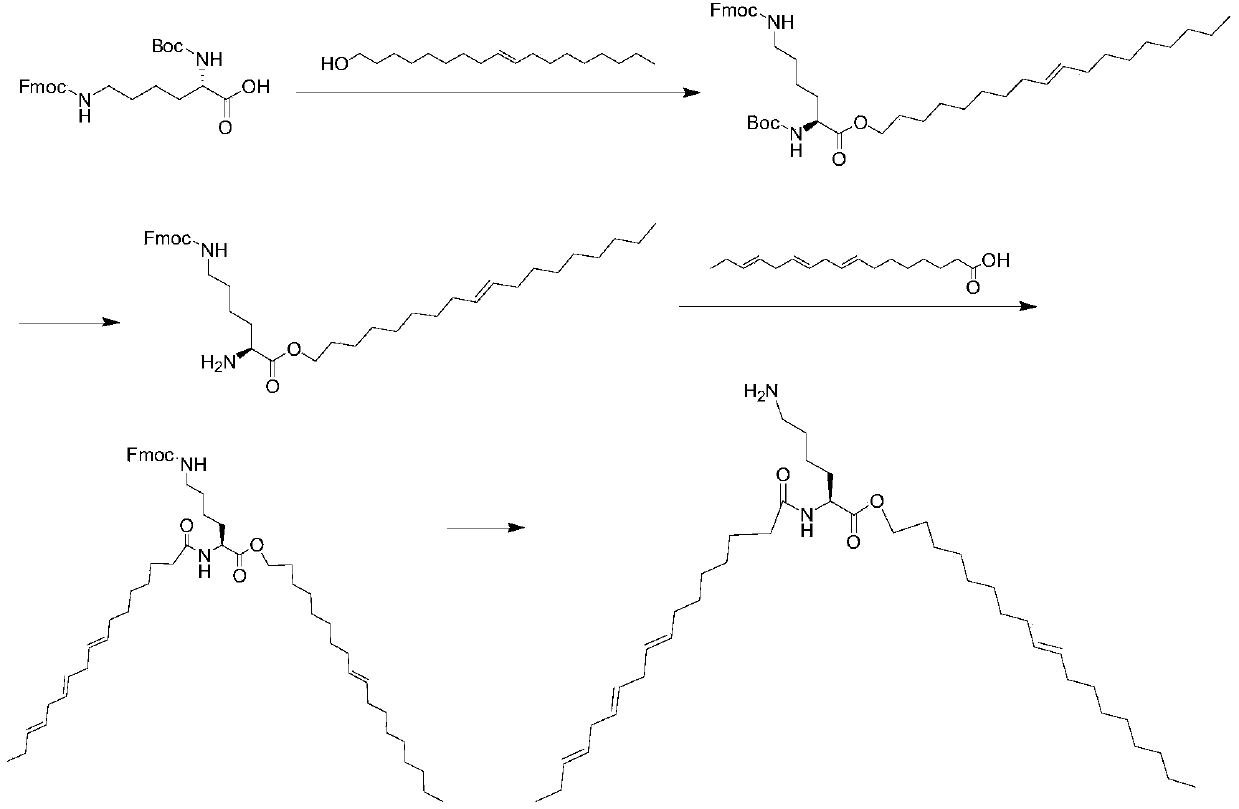
[0051] (2) figure 2 It is a schematic diagram of the synthesis method of ionizable cationic lipid L1;
[0052] (3) image 3 NMR spectrum results for ionizable cationic lipid L1;
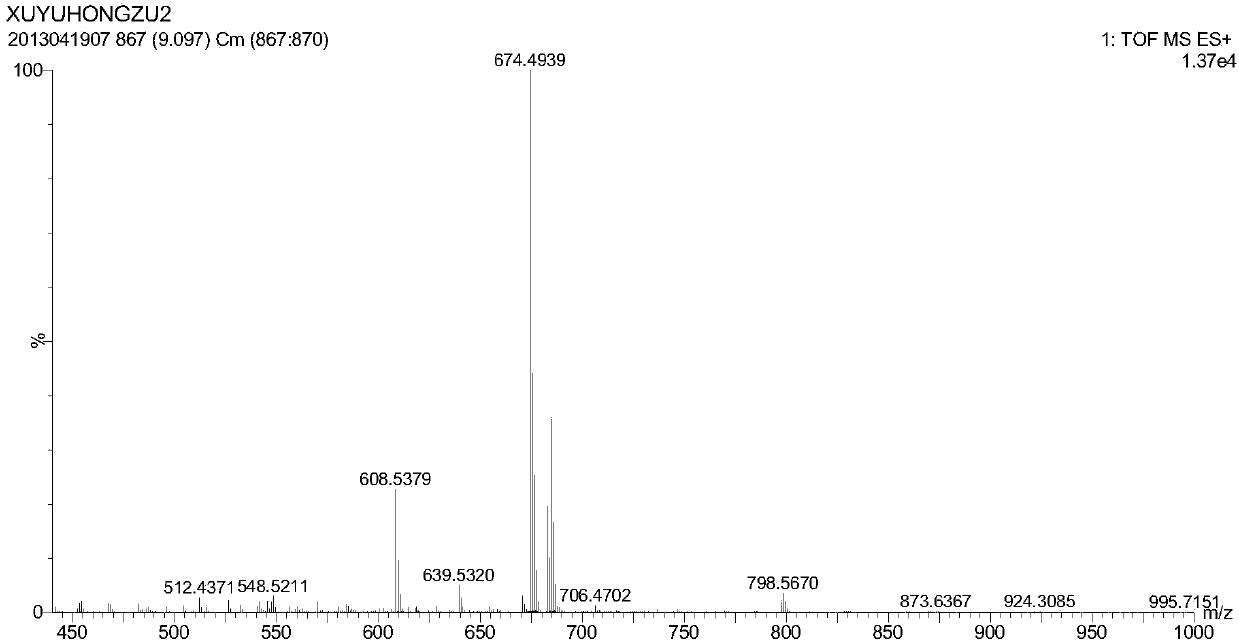
[0053] (4) Figure 4 Mass spectrometry analysis was performed for the ionizable cationic lipid L1, and the molecular weight of the obtained L1 lipid compound was close to the theoretical value, and the molecular weight was 658 g / mol.
Embodiment 2
[0054] Example 2, ionizable cationic lipid L2
[0055] Using lysine, linoleic acid, and dexamethasone as raw materials, L1 lipid was synthesized by Shanghai Maofu Chemical Technology Co., Ltd. according to the relevant technology. All of them have been purified by HPLC and identified by mass spectrometry. The purity is greater than 95%, and the molecular weight is consistent with the theoretical value.
[0056] Effect verification:
[0057] (1) Figure 5 is the structure diagram of ionizable cationic lipid L2;
[0058] (2) Image 6 Schematic diagram for the synthesis method of ionizable cationic lipid L2
[0059] (3) Figure 7 NMR spectrum results for ionizable cationic lipid L2;
[0060] (4) Figure 8 Mass spectrometry analysis was performed for the ionizable cationic lipid L2, and the molecular weight of the obtained L2 lipid compound was close to the theoretical value, and the molecular weight was 797 g / mol.
Embodiment 3
[0061] Example 3, L1 ionizable cationic liposomes
[0062] In this example, ionizable cationic liposomes were prepared according to different ratios using ionizable cationic lipid L1 and auxiliary lipid.
[0063] Its preparation method comprises the following steps:
[0064] 1. Preparation of Sample Solutions
[0065] Preparation of L1 ethanol solution, dipalmitoyl phosphatidyl choline (DPPC) ethanol solution, and cholesterol (Cholesterol, CHOL) ethanol solution: Weigh a certain amount with an electronic balance, add absolute ethanol to make it 10 mg / ml, and use it as stock solution;
[0066] Preparation of HEPES buffer (HEPES buffer): Weigh HEPES with an electronic balance, add deionized water, adjust pH with hydrochloric acid solution to make it 20mM pH 4.0, treat with diethyl pyrocarbonate (DEPC), and sterilize, use it as a stock solution;
[0067] 2. Preparation of liposomes:
[0068] 1) Take out L1 cationic lipid, cholesterol (CHOL), dipalmitoyl phosphatidylcholine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com