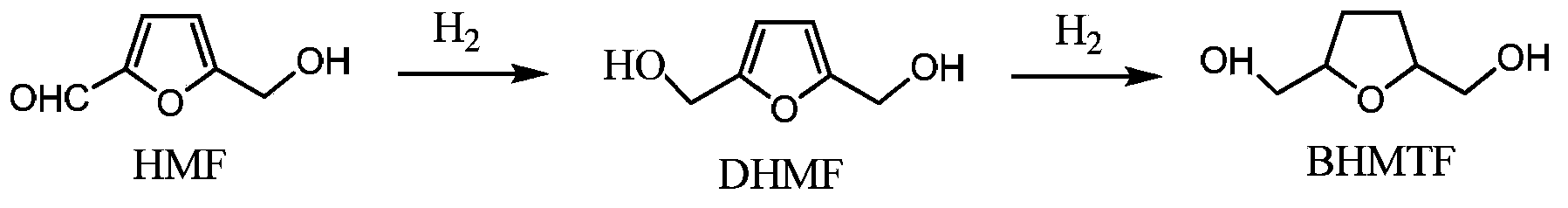
Method for preparing furyl glycol from fructosyl biomass
A kind of furanyl diol, biomass technology, applied in the direction of organic chemistry etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Supported metal catalysts with different loadings can use commercial catalysts or self-made by impregnation (Ji Na, Zhang Tao, Zheng Mingyuan, Wang Aiqin, Wang Hui, Wang Xiaodong, Chen Jingguang, Angew.Chem.Int.Ed.2008,47 ,8510-8513). Taking the preparation of Ni / C, Pd / C, Pt / C, Ir / C, Ru / C, Cu / C, Cu-Ru / C as an example: in the corresponding metal precursors nickel nitrate, palladium chloride, platinum chloride Aqueous solution of acid, chloroiridic acid, ruthenium trichloride, copper chloride, etc. impregnated the active carbon carrier with a medium volume, stirred evenly and dried at 60°C, then dried at 120°C for 12 hours, and finally dried at 450°C in a hydrogen atmosphere Reduction for 1 hour, cooling and passivation with 1% O2 / N2 mixed gas for 3 hours to obtain the catalyst.
Embodiment 2
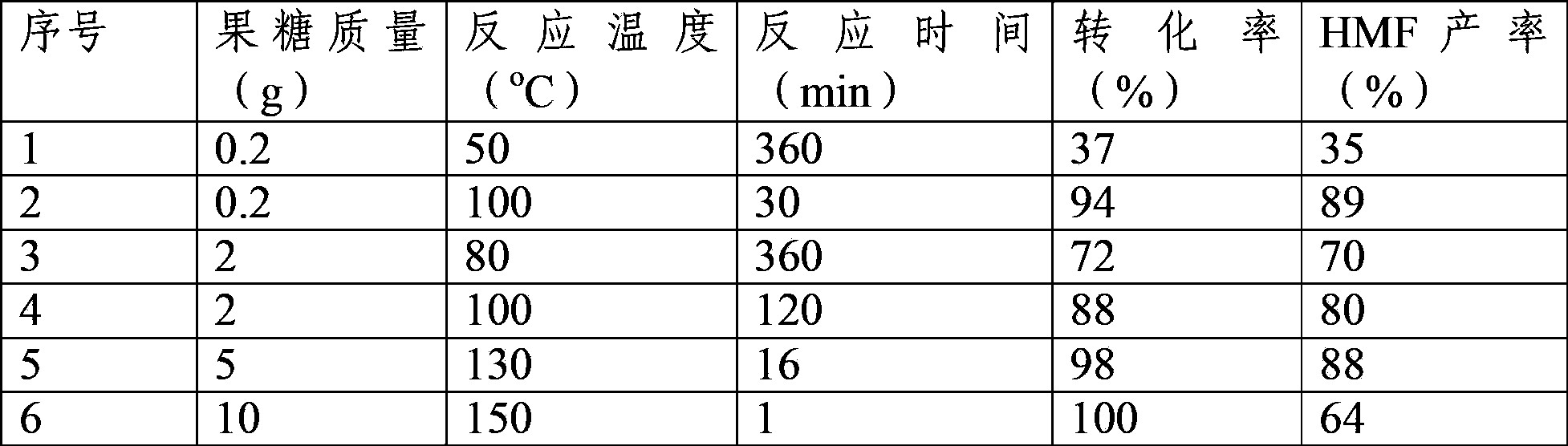
[0025] Weigh 2g of ionic liquid 1-butyl-3-methylimidazolium chloride (BmimCl) and a certain amount of fructose in a round bottom flask, heat and stir in oil baths at different temperatures, and regularly sample about 40mg (accurately weigh the mass and record ) and dilute to 1ml with deionized water, mix well and centrifuge, take the supernatant and analyze it with the external standard method of gas chromatography, the reaction results are shown in Table 1.
[0026] Table 1. The reaction results of HMF prepared from fructose dehydration in ionic liquid BmimCl
[0027]
[0028] The above results show that in the temperature range of 50°C-100°C, in the fructose / ionic liquid solution of 1:10-5:1 (wt / wt), fructose can be converted into HMF with a high yield, and the yield is 35 %-89%.
Embodiment 3
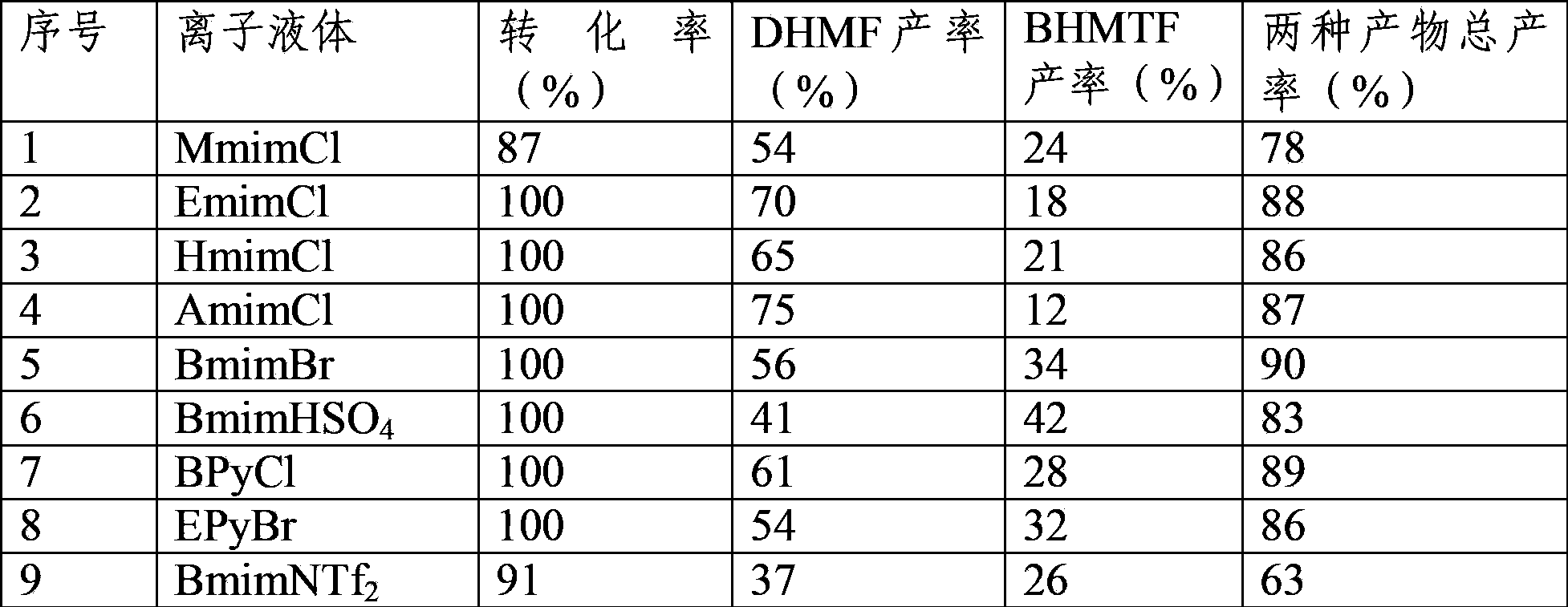
[0030] 0.5g of HMF, 2g of ionic liquid BmimCl and 70mL of water were added into the autoclave, and 0.15g of 5wt% Ru / C catalyst was added at the same time. Close the reactor, use H 2 Continuously replace the gas in the kettle for 5 times. then H 2Adjust the pressure to 5MPa, heat up to 50°C, stir rapidly (960rpm) for 6 hours, stop heating, and when the temperature of the kettle drops to room temperature, open the vent valve to reduce the pressure in the kettle to normal pressure, and then discharge. The reaction solution was filtered, and the filtrate was used for analysis. The product was qualitatively analyzed by GC-MS and quantitatively analyzed by GC external standard method. The HMF conversion rate was 100%, the DHMF yield rate was 74.3%, and the BHMTF yield rate was 20.1%. And up to 94.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com