A latex-enhanced immunoturbidimetric kit for quantitative detection of c-peptide
An immunoturbidimetric and latex-enhanced technology, which is applied in biological testing, measuring devices, material inspection products, etc., can solve the problems of unstable test results, high equipment requirements, and low degree of automation, so as to improve accuracy and credibility , reduce production costs, and achieve a high degree of automation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
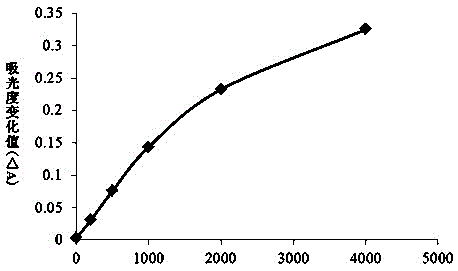
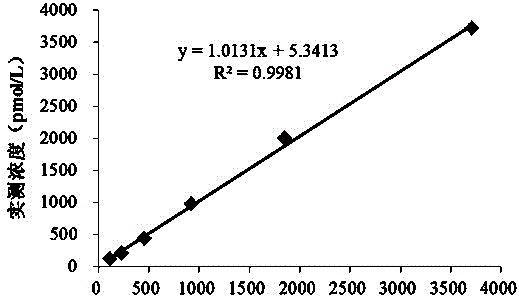
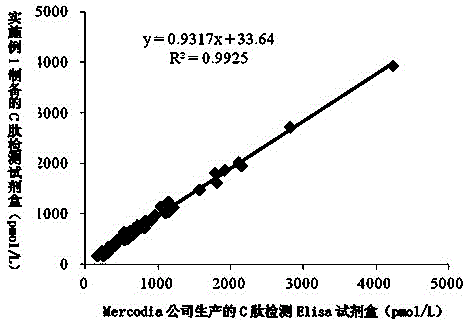
[0038] A latex-enhanced immunoturbidimetric kit for quantitative detection of C-peptide, including C-peptide R1 reagent, C-peptide R2 reagent and C-peptide calibrator.
[0039] Taking the preparation of 1LC peptide R1 reagent as an example, it is prepared from Tris-HCl buffer solution with a concentration of 50mM, 3g bovine serum albumin BSA, 1g sodium azide and 50g PEG6000, and the concentration of Tris-HCl buffer solution in the final C-peptide R1 reagent is 50mM, the concentration of bovine serum albumin BSA is 3g / L, the concentration of sodium azide is 1g / L, and the concentration of PEG6000 is 50g / L;
[0040] Taking the preparation of 1LC peptide R2 reagent as an example, it is prepared from Tris-HCl buffer solution with a concentration of 50mM, 5g of bovine serum albumin BSA, 1g of sodium azide and sensitized polystyrene latex particles coated with anti-human C-peptide antibody Obtained, the concentration of Tris-HCl buffer solution in the final C peptide R2 reagent is 50...
Embodiment 2
[0056] A latex-enhanced immunoturbidimetric kit for quantitative detection of C-peptide, including C-peptide R1 reagent, C-peptide R2 reagent and C-peptide calibrator.
[0057] Taking the preparation of 1LC peptide R1 reagent as an example, it is prepared from 200mM glycine buffer, 5g bovine serum albumin BSA, 2g sodium azide and 40g PEG8000. The concentration of glycine buffer in the final C peptide R1 reagent is 200mM, bovine serum albumin The concentration of protein BSA is 5g / L, the concentration of sodium azide is 2g / L, and the concentration of PEG8000 is 40g / L;
[0058] Taking the preparation of 1LC peptide R2 reagent as an example, it is prepared from 50mM borax buffer solution, 5g bovine serum albumin BSA, 0.3g sodium azide and sensitized polystyrene latex particles coated with anti-human C-peptide antibody , the concentration of borax buffer in the final C-peptide R2 reagent is 50mM, the concentration of bovine serum albumin BSA is 5g / L, and the concentration of sodiu...
Embodiment 3
[0062] A latex-enhanced immunoturbidimetric kit for quantitative detection of C-peptide, including C-peptide R1 reagent, C-peptide R2 reagent and C-peptide calibrator.
[0063] Taking the preparation of 1LC peptide R1 reagent as an example, it is prepared from PBS buffer solution with a concentration of 200mM, 5g bovine serum albumin BSA, 3g sodium azide and 50g PEG8000, the final concentration of PBS buffer solution in the C peptide R1 reagent is 200mM, bovine serum The concentration of albumin BSA is 5g / L, the concentration of sodium azide is 3g / L, and the concentration of PEG8000 is 50g / L;
[0064] Taking the preparation of 1LC peptide R2 reagent as an example, it is prepared from borax buffer solution with a concentration of 50mM, 5g of bovine serum albumin BSA, 1g of sodium azide, and sensitized polystyrene latex particles coated with anti-human C-peptide antibody. The concentration of borax buffer in the final C-peptide R2 reagent is 50mM, the concentration of bovine ser...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com