A kind of isradipine formula medicine tablet and application thereof
A technology of isradipine and drugs, applied in the field of combination drugs, can solve the problems of slow effect and long duration, etc., and achieve good active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The screening of embodiment 1 isradipine prescription drug tablet preparation conditions
[0014] 1. Screening of the types of disintegrants
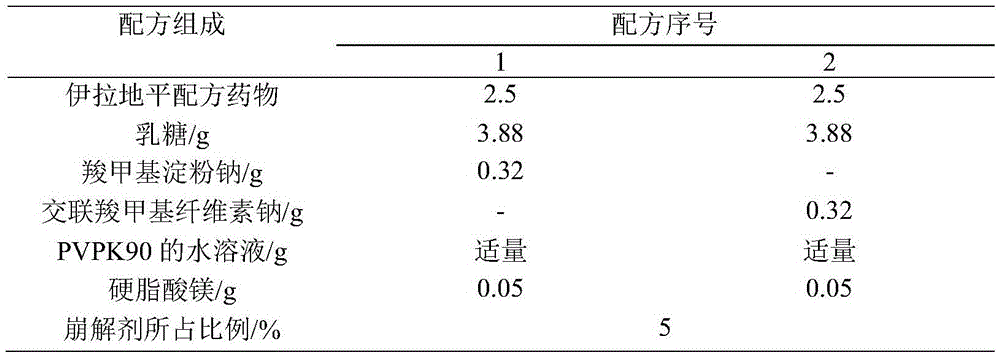
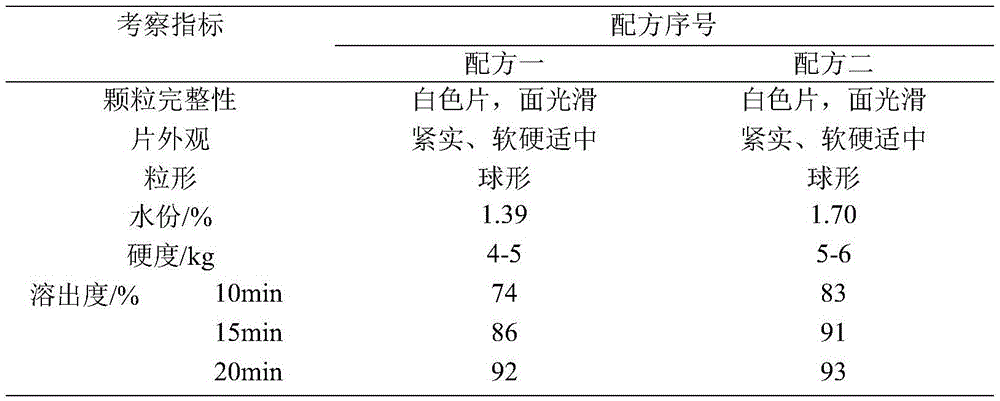
[0015] Taking the particle shape, water content of the granules, the hardness of the plain tablet, and the dissolution curve as the investigation indicators, the dosage of the disintegrant was fixed at 5%, and the disintegrants sodium carboxymethyl starch and croscarmellose sodium were investigated. The formula is shown in Table 1, and the test results are shown in Table 2.
[0016] Table 1 Screening of the types of disintegrants (formulation quantity 50 tablets)
[0017]
[0018] Preparation method: Weigh the raw and auxiliary materials of the formula respectively, mix them evenly, add an appropriate amount of binder to make a suitable soft material, pass through a 30-mesh sieve to granulate, dry at (50±5)°C until the water content is less than 3.0%, and use a 30-mesh Sieve for granulation, add magnesium stearate, mix and c...
Embodiment 2
[0033] The preparation of embodiment 2 isradipine formula medicine tablet
[0034] Adopting is the preferred formula conclusion of embodiment 1, according to the formula described in table 6, take the raw and auxiliary materials of the prescription amount, mix uniformly, add an appropriate amount of 4% binder to make a suitable soft material, cross 30 mesh sieves for granulation, and Dry at 50±5)°C to moisture <3.0%, granulate with a 30-mesh sieve, add magnesium stearate, mix and compress into tablets, and spray; gastric-soluble film coating premix Opadry (295W680000, containing hypromellose Cellulose, polyethylene glycol, titanium dioxide), namely the drug test group I.
[0035] Formulation 1 of table 6 isradipine formulation drug tablet
[0036]
[0037] The raw materials were weighed according to the formula in Table 7, and the drug experiment group II was prepared by the above method.
[0038] Formulation 2 of table 7 isradipine formulation drug tablet
[0039]
...
Embodiment 3
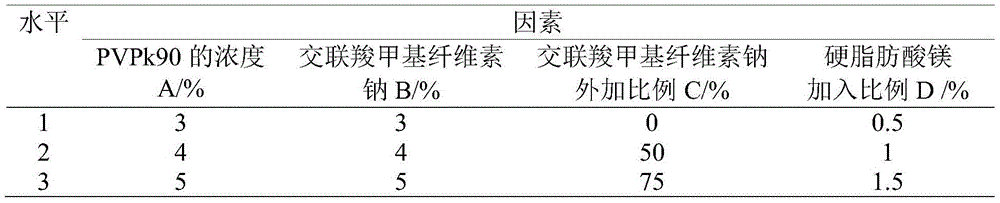
[0043] Embodiment 3 Experimental drug is investigated to the activity of hypertension
[0044] 10 male Wistar-Kyoto (WKY) rats of SFP grade 6-8 weeks old, 60 male SHR rats of SFP grade 6-8 weeks old. )°C, relative humidity (55-4-5)%, free access to food and water, pre-raised for 1 week.
[0045] Grouping of Experimental Animals 50 SHRs were randomly divided into 6 groups, namely model control group, isradipine group, quercetin group and drug experimental group Ⅰ, Ⅱ, Ⅲ, and 10 Wistar-Kyoto rats were used as normal control (WKY) Group. Experimental animals in all groups were subjected to a forced gavage administration method, and the administration volume was 1ml / 100g body weight.
[0046] Dosage: the model control group and the normal control group were administered with normal saline, the isradipine group was administered with 50 mg / kg of isradipine, the quercetin group was administered with 50 mg / kg of isradipine, and the drug experimental group was administered with 50 mg / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com