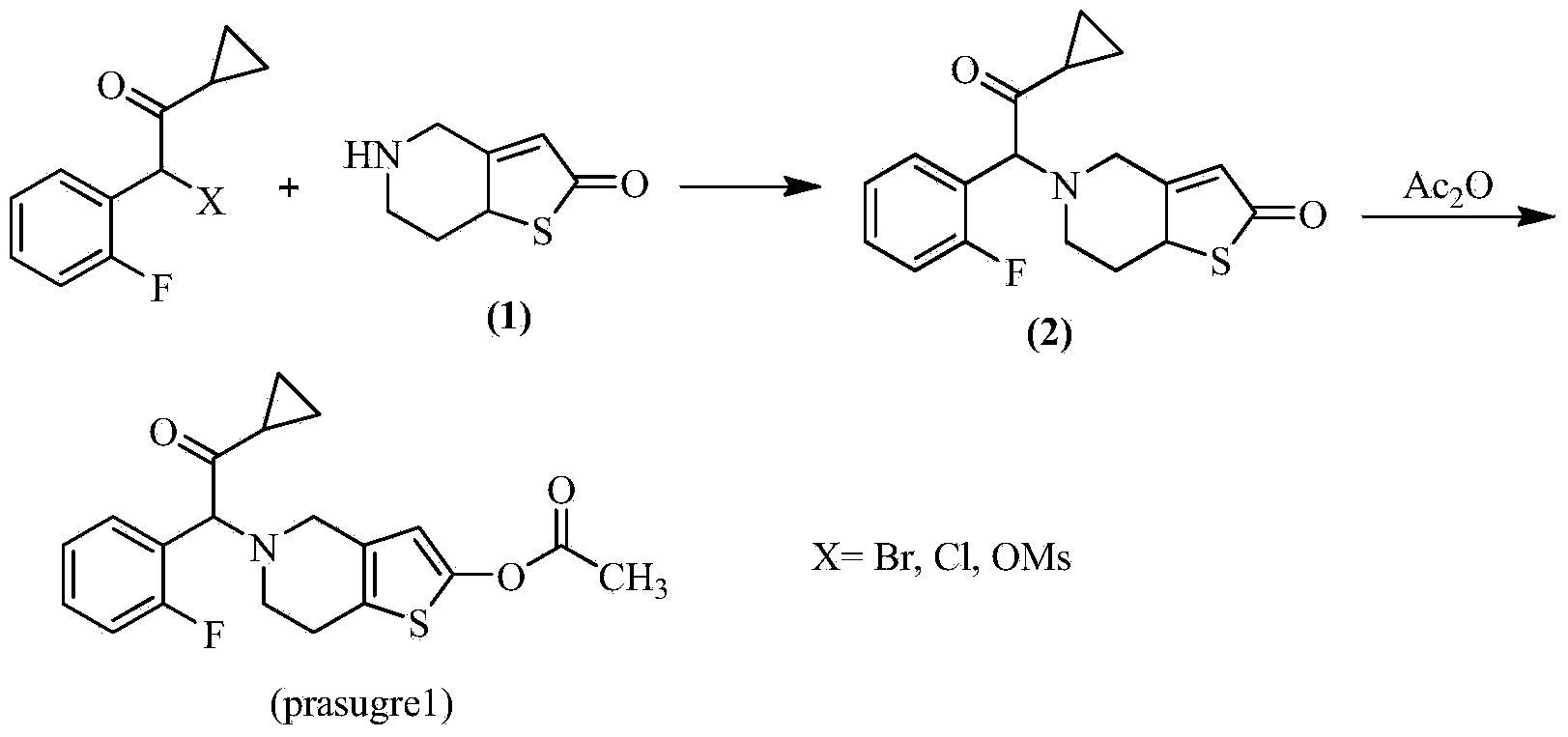
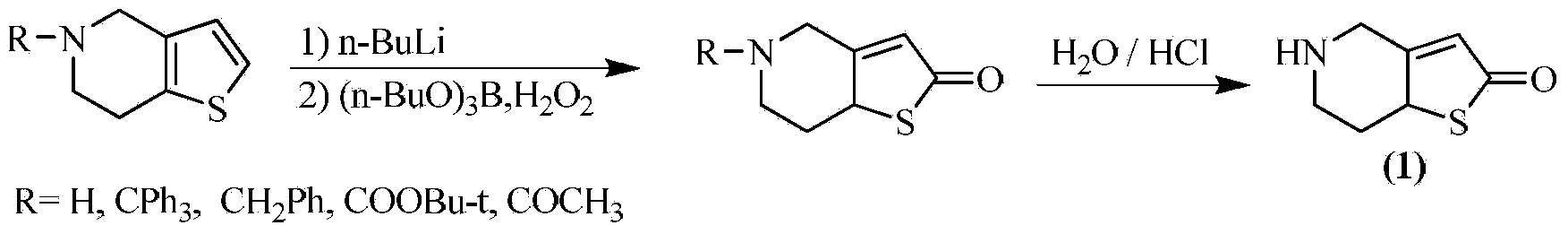
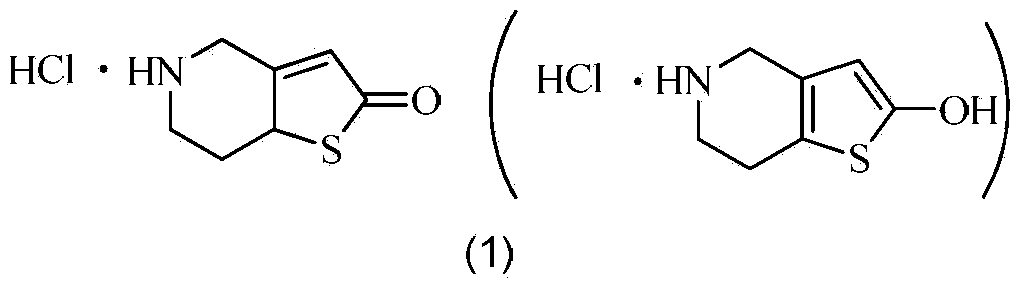Preparation method of thienopyridine compound
A technology of phenopyridine and thiophene, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of complicated operation, poor reaction reproducibility, long reaction time and the like, and achieves the effects of simple operation process, mild reaction conditions and easy-to-obtain production raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of 2,5-diacetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0032]
[0033] Add 18.2g (0.1mol) of 5-acetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine and 13.4g (0.1mol) of anhydrous aluminum trichloride into a 250mL reaction flask and 80mL of dichloromethane, cooled to -15°C, 7.8g (0.1mol) of acetyl chloride was added dropwise under stirring, after the drop was complete, the reaction was stirred and reacted at the same temperature for 2 hours, and then raised to room temperature and continued to stir and react for 2 hours (TLC monitored the reaction end point, Developing agent: petroleum ether / ethyl acetate=1 / 1). The reaction solution was poured into 100 g of crushed ice, the organic layer was separated, the aqueous layer was extracted with dichloromethane (30 mL×3), the organic phases were combined, dried over anhydrous sodium sulfate, the desiccant was filtered off, concentrated under reduced pressure to recover two Chloromethane, the residue was...
Embodiment 2
[0034] Example 2: Preparation of 2,5-diacetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0035]
[0036] Add 18.2g (0.1mol) of 5-acetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine, 9.42g (0.12mol) of acetyl chloride and 80mL of dichloro Methane, mixed evenly, cooled to -5°C, stirred for 30min, added dropwise the mixed solution composed of 31.3g (0.1mol) anhydrous tin tetrachloride and 70mL dichloromethane, after dropping, stirred and reacted at the same temperature for 4 hours ( The end point of the reaction was monitored by TLC, developing solvent: petroleum ether / ethyl acetate=1 / 1). The reaction solution was poured into 100 g of crushed ice containing 1 mL of concentrated hydrochloric acid, the organic layer was separated, the aqueous layer was extracted with dichloromethane (30 mL × 3), the organic phases were combined, dried over anhydrous sodium sulfate, and the desiccant was filtered off. Dichloromethane was recovered by concentration under reduced pressure, and the residue...
Embodiment 3
[0037] Example 3: Preparation of 2-acetylamino-5-acetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine
[0038]
[0039] Add 11.2g (0.05mol) 2,5-diacetyl-4,5,6,7-tetrahydrothieno[3,2-c]pyridine, 11.1g (0.11mol) triethylamine into a 250mL reaction flask , 60mL absolute ethanol. 7.3g (0.11mol) of hydroxylamine hydrochloride was dissolved in 10mL of water, and the aqueous solution of hydroxylamine hydrochloride was added dropwise to the reaction system under an ice bath. After the dropping was complete, the mixture was refluxed for 4 hours, and a white solid was precipitated during the reaction. Filtrate with suction, wash the filter cake with water, and dry to obtain a white solid, which is the hydroxime intermediate, which is hydrolyzed without purification.
[0040] Mix 6.3g (0.025mol) of the prepared hydroxime intermediate with 80mL tetrahydrofuran evenly, quickly add 6.2g (0.03mol) phosphorus pentachloride under ice bath cooling, stir and react at the same temperature for 3 hou...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Yield | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com