Biodegradable hemostatic membrane
A kind of hemostatic film, biological technology, applied in absorbent pads, medical science, bandages, etc., to achieve excellent hemostasis, wide application range, and little pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
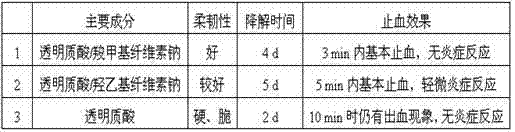
[0035] In order to investigate the effect of cellulose on the efficacy of the hemostatic film, in this example, hyaluronic acid / sodium carboxymethyl cellulose, hyaluronic acid / sodium hydroxyethyl cellulose and pure hyaluronic acid were prepared respectively as the main components. the hemostatic film, and compare the differences among the three.
[0036] (1) Use NaOH to adjust the pH of deionized water to 10~12, add 1,4-butanediol diglycidyl ether, and mix well to obtain a mixed solution;
[0037] (2) Three groups of gels were prepared: No. 1, 1000 kD sodium hyaluronate and 500 kD sodium carboxymethyl cellulose were dissolved in the mixed solution, and the mass ratio of sodium hyaluronate to sodium carboxymethyl cellulose was 4 : 1. Mix evenly and fully dissolve the biological polysaccharide to prepare gel I with both sodium hyaluronate and sodium carboxymethyl cellulose concentration of 10%. The amount of 1,4-butanediol diglycidyl ether added accounts for the 1.25% of the to...
Embodiment 2
[0044] (1) Use NaOH to adjust the pH of deionized water to 10~12, add 1,4-butanediol diglycidyl ether, and mix well to obtain a mixed solution;
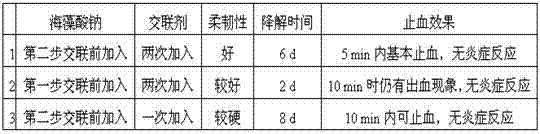
[0045] (2) Three groups of gels were prepared, the mass ratio of sodium hyaluronate and sodium carboxymethylcellulose was 2:1, and the mass ratio of sodium hyaluronate and sodium alginate was 1:0.1; The amount of 4-butanediol diglycidyl ether added is 1.25% of the total weight of sodium hyaluronate and sodium carboxymethyl cellulose: No. 1 Dissolve 1500 kD sodium hyaluronate and 500 kD sodium carboxymethyl cellulose In the mixed solution, mix evenly and fully dissolve the biological polysaccharide to prepare gel I with a sodium hyaluronate concentration of 10% and a sodium carboxymethyl cellulose concentration of 5%. Gel II with 1500 kD 1% sodium alginate and cross-linking agent dissolved, the amount of 1,4-butanediol diglycidyl ether in gel II is half of that in gel I, and the pH is adjusted to 3 ~5, 35℃ for 8 h, purified and freez...
Embodiment 3
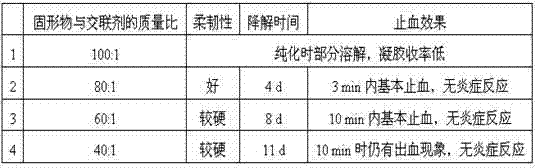
[0052] In this example, the influence of different molecular weight hyaluronic acid on its physicochemical properties and hemostatic application effect in the preparation of hemostatic film was investigated.
[0053] (1) Use 0.25 N NaOH to adjust the pH of deionized water to 10~12, add 1,4-butanediol diglycidyl ether, and mix well to obtain a mixed solution;
[0054] (2) Dissolve sodium hyaluronate and sodium carboxymethyl cellulose with molecular weights of 500 kD, 1000 kD, 1500 kD, 2000 kD and 500 kD in the mixed solution, respectively. The mass ratio is 2:1, 1,4-butanediol diglycidyl ether accounts for 1.25% of the total weight of sodium hyaluronate and sodium carboxymethyl cellulose, mix well and fully dissolve the biological polysaccharide to prepare sodium hyaluronate Gel I with a concentration of 10% was subjected to alkaline crosslinking at 35 °C for 4 h;
[0055] (3) Add 500 kD sodium alginate into deionized water mixed with 1,4-butanediol diglycidyl ether, mix well ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com