Angiotensin-converting enzyme inhibition peptide sourcing from marine microalgae
An angiotensin and inhibitory peptide technology, applied in the field of active peptide screening, to achieve the effect of novel structure, small molecular weight and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Separation and purification of angiotensin-converting enzyme inhibitory peptide
[0021] (1) Preparation of Isochrysis globosa protein:
[0022] Add Isoflagellates to 1.5% (w / v) sodium chloride solution at a ratio of 1:15 (1 gram of algae powder is added to 15 ml of sodium chloride solution), adjust the pH to 7.0, extract with ultrasonic waves, and crush at high speed Refrigerate and centrifuge at 4°C and 5000r / min for 15min, centrifuge to separate the supernatant, add sodium chloride solution to the precipitated algal mud and ultrasonically crush it twice, with an interval of 1s, combine all the supernatant obtained after the above crushing, and freeze-dry That is crude protein.
[0023] (2) Enzymolysis:
[0024] With the crude protein in step (1), according to the enzyme and crude protein ratio of 6:100, add trypsin with an enzyme activity of 10000U / g, adjust the pH of the solution to 8.0, and the concentration of the substrate protein to be 5.5g / 100ml. T...
Embodiment 2
[0033] Example 2 Sequence Analysis of Angiotensin Converting Enzyme Inhibitor Peptide
[0034] Taking the angiotensin-converting enzyme inhibitory peptide prepared in Example 1, the amino acid composition of the active polypeptide was first analyzed, and the results showed that its main amino acids were Met, Lys, and Leu, and it also contained Tyr, Gly, and Asp. Using MALDI-TOF / TOF-MS / MS to analyze the amino acid sequence of the polypeptide, the molecular weight of the active polypeptide was analyzed to be 839.4061Da, and the determined amino acid sequence was: Tyr-Met-Gly-Leu-Asp-Leu-Lys. De novo sequencing analysis was performed on the structure, and the results were consistent with the above data, confirming that the polypeptide is Tyr-Met-Gly-Leu-Asp-Leu-Lys (SEQ ID NO: 1), which is a new type of active polypeptide structure.
Embodiment 3
[0035] Example 3 Effect of angiotensin-converting enzyme inhibitory peptide
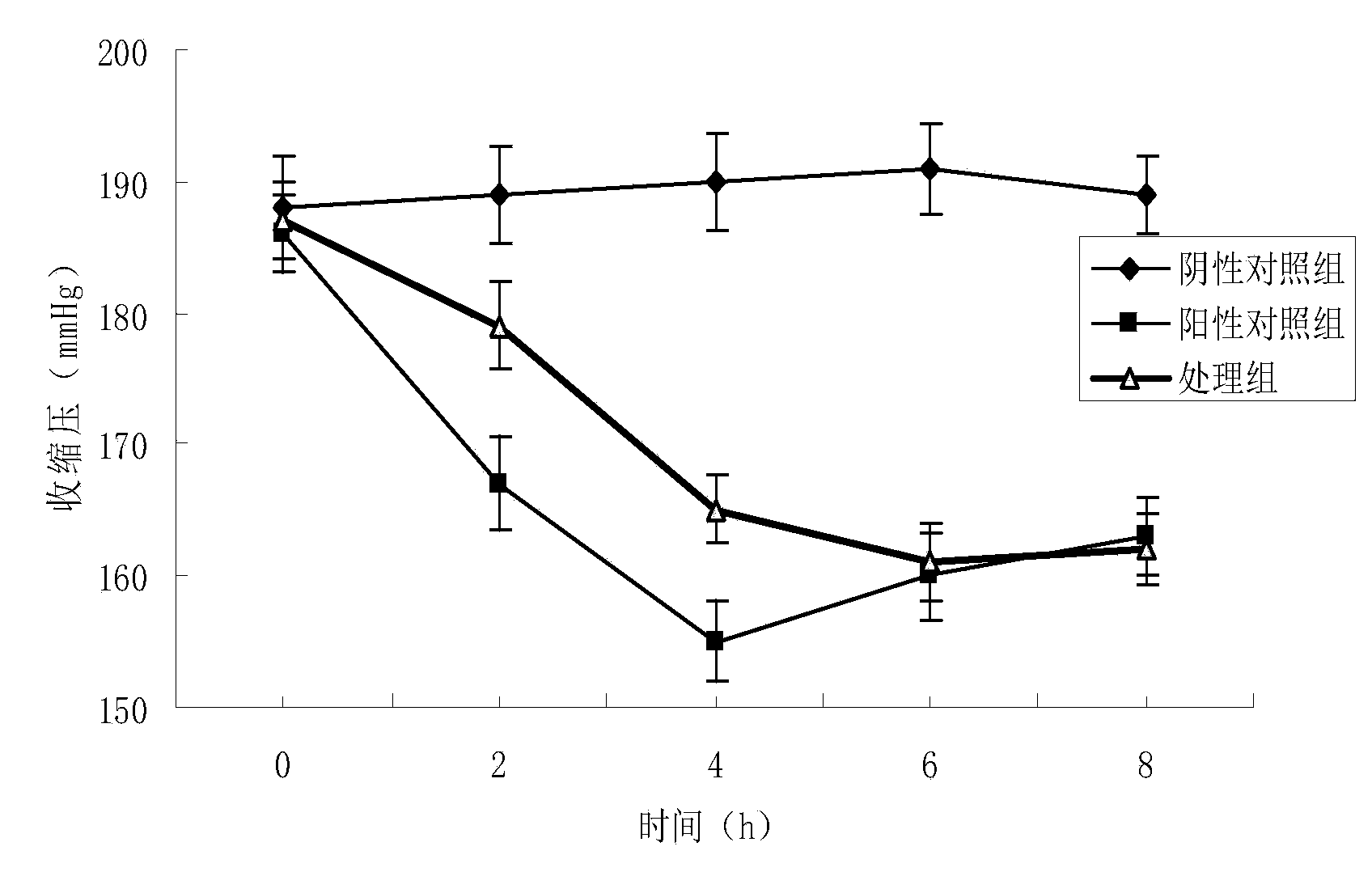
[0036] The antihypertensive peptide derived from marine microalgae obtained in Example 1 was directly administered into essential hypertensive rats (SHR), and its effect on the blood pressure of SHR was detected. The experiment selected 24 rats with a body weight of 240 ± 20 grams. The rats were randomly divided into 3 groups, 8 in each group, including a blank control group (injection of the same volume of normal saline), an ACE enzyme inhibition group and a synthetic antihypertensive drug card. Positive control group for Topril. The experimental temperature was 20°C, the relative humidity was 70%, and the pre-feeding was carried out for 1 week. The administration dose was 10 mg / kg, and the time was 24 days. The systolic pressure of the tail artery was measured once at 0, 2, 4, 6, and 8 hours. The results showed that the ACE inhibitory peptide group had a significant effect compared with the blank...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com