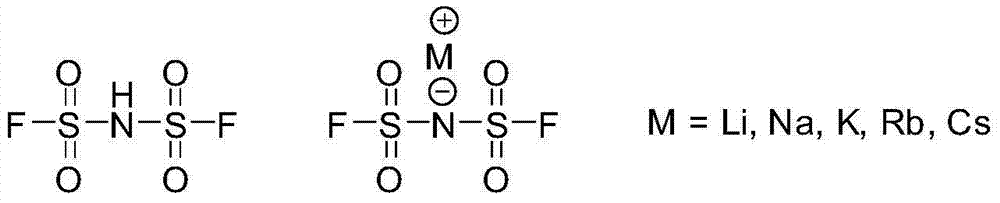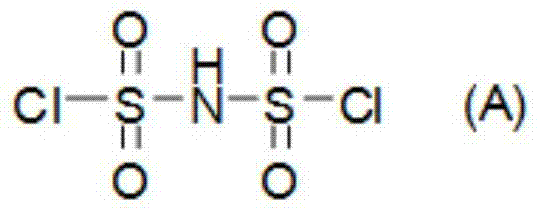Preparation methods of bis(fluorosulfonyl)imide and alkali metal salts thereof
The technology of bisfluorosulfonimide base and bisfluorosulfonimide is applied in the field of preparation of fluorine-containing compounds, which can solve the problems of high manufacturing cost, long process flow and high raw material cost, and achieves reduction of material consumption and waste. Generate, simplify the preparation process, reduce the effect of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
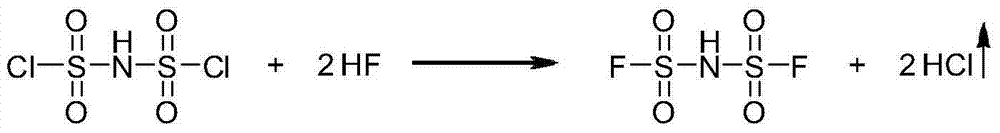
[0030] Specifically, the preparation method of bisfluorosulfonimide provided by the present invention includes the step of preparing bisfluorosulfonimide by reacting bischlorosulfonimide represented by structural formula (A) with hydrogen fluoride.
[0031]
[0032] Wherein, the chemical reaction equation that takes place in the preparation process of bisfluorosulfonimide is as follows:
[0033]
[0034] Preferably, the dichlorosulfonimide is prepared by the mixed reaction of sulfonamide, thionyl chloride and chlorosulfonic acid, and the reaction equation is as follows:
[0035]
[0036] Wherein, the reaction temperature is controlled at 120-140° C., and the reaction time is 20-30 hours. Acid gas (SO 2 , HCl) can be passed into the lye for absorption, and the dichlorosulfonimide obtained after the reaction can be further purified by vacuum distillation. The dichlorosulfonimide fraction temperature is 110-114°C / 2mmHg.
[0037] Preferably, the molar ratio of the sulfo...
Embodiment 1
[0052] Embodiment 1: Preparation of bisfluorosulfonimide
[0053] Under stirring, 291 g of sulfonamide (3 mol), 1071 g of thionyl chloride (9 mol), and 349.5 g of chlorosulfonic acid (3 mol) were successively added into a dry 2L reaction vessel to obtain a mixed solution. Heat the mixed solution to 120°C for reaction, and the sulfur dioxide and hydrogen chloride acid gas produced will be absorbed by the lye. The brownish-yellow liquid crude product obtained after 30 hours of reaction was vacuum-distilled under reduced pressure, and the fraction at 110-114°C / 2mmHg was collected to obtain 610 g of dichlorosulfonimide colorless liquid, with a yield of 95%;
[0054] Under constant temperature stirring at 10°C, add 400 grams of hydrogen fluoride (20 mol) into a 1L dry reaction vessel, and slowly add 428 grams of bischlorosulfonimide (2 mol) for fluorination reaction, and the generated hydrogen chloride acid gas is absorbed by the lye. After the dropwise addition of dichlorosulfoni...
Embodiment 2
[0055] Example 2: Preparation of lithium bisfluorosulfonyl imide
[0056] Under stirring, 679 g of sulfonamide (7 mol), 1785 g of thionyl chloride (15 mol), and 815.5 g of chlorosulfonic acid (7 mol) were successively added into a dry 5 L reaction vessel to obtain a mixed solution. Heat the mixed solution to 140°C for reaction, and the sulfur dioxide and hydrogen chloride acid gas produced will be absorbed by the lye. After reacting for 20 hours, the brownish-yellow liquid crude product obtained was vacuum-distilled under reduced pressure, and the fraction at 110-114°C / 2mmHg was collected to obtain 1438 g of dichlorosulfonimide colorless liquid, with a yield of 96%.
[0057] Under constant temperature stirring at 5°C, add 1600 grams of hydrogen fluoride (80 mol) and 156 grams of lithium fluoride (6 mol) into a 3L dry reaction vessel, slowly add 1284 grams of bischlorosulfonimide (6 mol) for chemical reaction, and produce hydrogen chloride Acid gases are absorbed by lye. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com