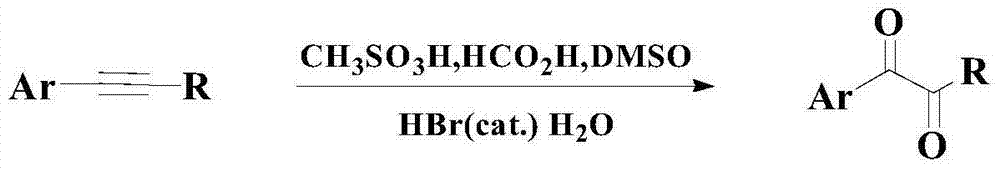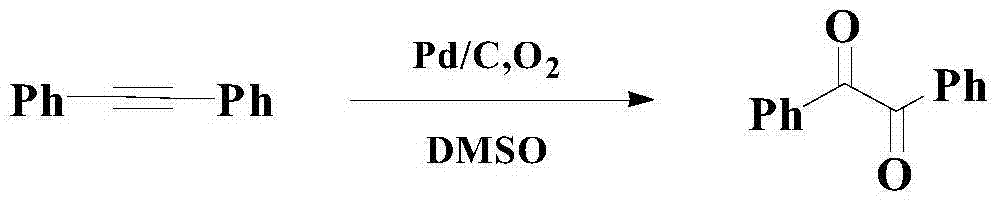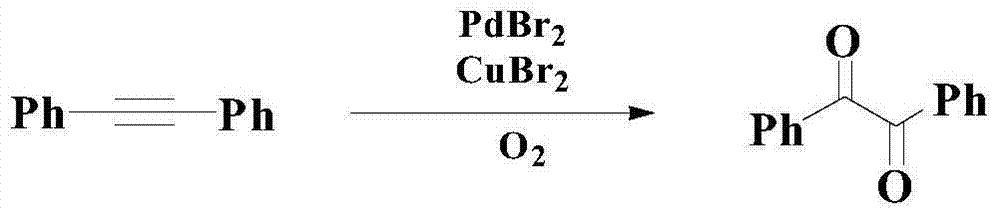Catalytic synthesis method of organic chemical intermediate 1,2-diketone compound
A synthesis method and compound technology are applied in the fields of catalytic synthesis of organic chemical intermediates 1,2-diketone compounds and catalytic oxidation of aryl alkyl alkynes to prepare 1,2-diketone compounds, which can solve the problem of narrow bottom The scope of application of chemical substances, inability to fully meet the problems, low reaction yield and chemical selectivity, etc., to overcome the poor catalytic performance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] Add 1mmol formula (I) compound and 12ml acetone in the reactor at room temperature, then slowly add 2.2mmol mercuric acetate and 2.2mmol water, then add 70mg of ferrocene with a mass ratio of 0.2:0.25:1 while stirring , salicylic acid and triethyloxonium tetrafluoroborate additive mixture, after mixing, continue to stir the reaction for 0.5h, monitor the reaction end point by TLC, add an equal volume of water to the system after the reaction is completed, and then use ethyl acetate The ester was extracted three times, the organic phase was combined, and the organic phase was washed with 1 / 2 volume of saturated brine, the organic phase was separated, dried by adding anhydrous sodium sulfate, filtered, and finally the organic solvent was removed by vacuum distillation, and the residue was purified by silica gel column chromatography , the compound of formula (II) can be obtained with a yield of 98.6% and a purity of 99.1% (HPLC).
[0033] 1 H NMR (500MHz, CDC...
Embodiment 2
[0035]
[0036] Add 1mmol formula (I) compound and 15ml acetone in reactor at room temperature, then slowly add 2.5mmol mercuric acetate and 2.5mmol water, then add 62mg of ferrocene in a mass ratio of 0.2:0.25:1 while stirring , salicylic acid and triethyloxonium tetrafluoroborate additive mixture, after mixing, continue to stir the reaction for 1.5h, monitor the reaction end point by TLC, add an equal volume of water to the system after the reaction is completed, and then use ethyl acetate The ester was extracted three times, the organic phase was combined, and the organic phase was washed with 1 / 2 volume of saturated brine, the organic phase was separated, dried by adding anhydrous sodium sulfate, filtered, and finally the organic solvent was removed by vacuum distillation, and the residue was purified by silica gel column chromatography , the compound of formula (II) can be obtained with a yield of 98.8% and a purity of 99.3% (HPLC).
[0037] 1 H NMR (400MHz, CDCl 3 ) δ...
Embodiment 3
[0039]
[0040] Add 1mmol formula (I) compound and 13ml acetone in reactor at room temperature, then slowly add 2.3mmol mercuric acetate and 2.3mmol water, then add 65mg of ferrocene with a mass ratio of 0.2:0.25:1 while stirring , salicylic acid and triethyloxonium tetrafluoroborate additive mixture, after mixing, continue to stir the reaction for 2h, monitor the reaction end point by TLC, add an equal volume of water to the system after the reaction is completed, and then use ethyl acetate Extract three times, combine the organic phase, add 1 / 2 volume of saturated brine to wash the organic phase, separate the organic phase, add anhydrous sodium sulfate to dry, filter, and finally remove the organic solvent by vacuum distillation, and the residue is purified by silica gel column chromatography. The compound of formula (II) can be obtained with a yield of 98.9% and a purity of 99.2% (HPLC).
[0041] 1 H NMR (500MHz, CDCl 3 )δ=7.94(d, J=8.8Hz, 2H), 6.92(d, J=8.8Hz, 2H), 6....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com