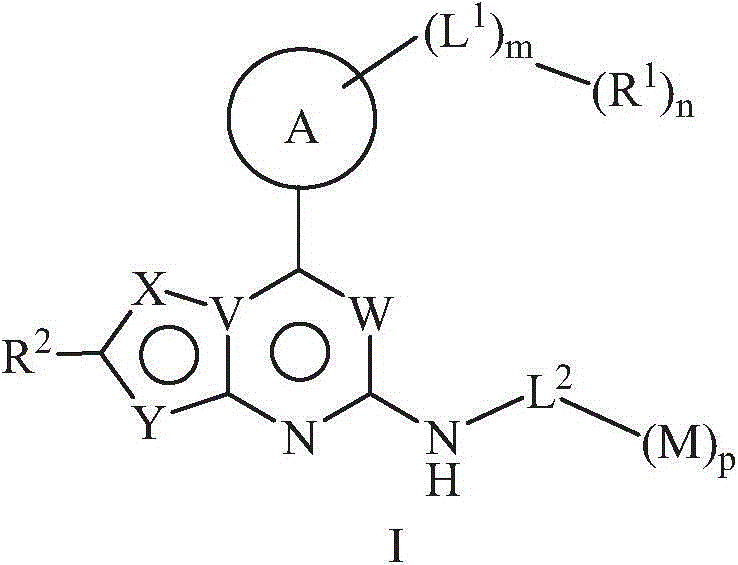
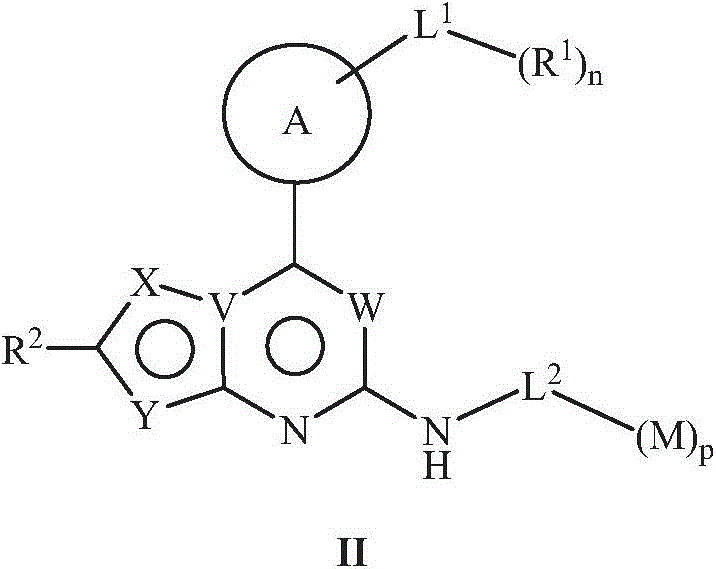
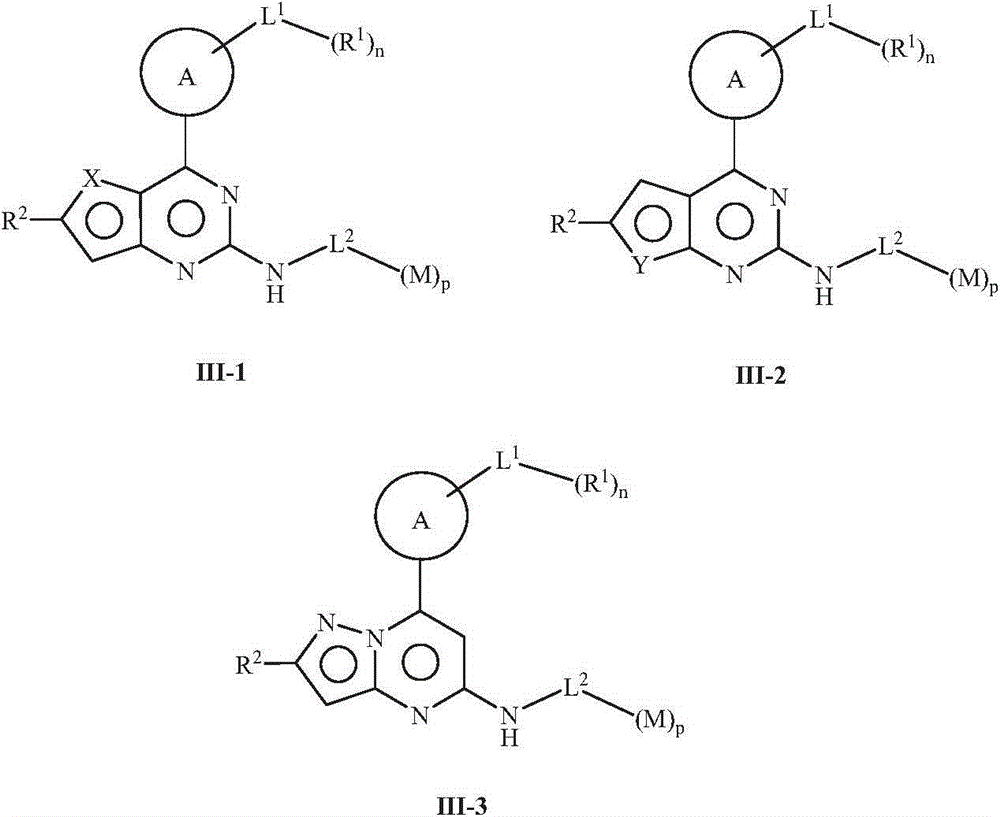
5-member and 6-member rings heterocyclic compound, its preparation method, pharmaceutical composition and its application
A six-membered heterocyclic compound technology, applied in the field of five-membered and six-membered heterocyclic compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0138] Example 1 3-{4-[2-(Benzylamino)thieno[3,2-d]pyrimidin-4-yl]-1H-pyrazol-1-yl}butyronitrile T-01
[0139] The synthetic route is as follows:
[0140]
[0141] Synthesis of compound 1-a
[0142] Cesium carbonate (13.3g, 41.2mmol) was added to a solution of 3-bromobutylcyanide (2.0g, 10.3mmol) and 4-pyrazole borate pinazolate (2.3g, 15.5mmol) in acetonitrile (100mL), and the mixture Heat to 90°C and stir for 3 hours. After cooling to room temperature, water (100 mL) was added to quench the reaction. Extracted with ethyl acetate (100mL×3), the organic phases were combined, washed with water (60mL×3) and saturated brine (60mL) successively, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a colorless liquid 1- a (2.3g), the crude product was directly put into the next reaction. LC-MS(ESI): m / z=262[M+H] + .
[0143] Synthesis of compound 1
[0144] Under nitrogen atmosphere, sodium carbonate (318 mg, 3....
Embodiment 2
[0148] Example 2 3-{4-[2-(phenylamino)thieno[3,2-d]pyrimidin-4-yl]-1H-pyrazol-1-yl}butyronitrile T-02
[0149] The synthetic route is as follows:
[0150]
[0151] Compound 1 (30mg, 0.1mmol), aniline (55mg, 0.6mmol) and p-toluenesulfonic acid monohydrate (76mg, 0.4mmol) were dissolved in isobutanol (8mL), and the mixture was stirred at 110°C for 16 hours . The reaction solution was concentrated under reduced pressure, and the residue was diluted with ethanol (30 mL), washed successively with saturated aqueous sodium bicarbonate (30 mL), water (30 mL) and saturated brine (30 mL), dried over anhydrous sodium sulfate, filtered, and reduced pressure The filtrate was concentrated, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=1:1) to obtain compound T-02 (20 mg, yield: 55%). LC-MS(ESI): m / z=361[M+H] + .
[0152] 1 HNMR (400MHz, CDCl 3 )δ: 8.35(s, 1H), 8.32(s, 1H), 7.88(d, J=5.6Hz, 1H), 7.75(d, J=8.0Hz, 1H), 7.33~7.39(m, 4H)...
Embodiment 3
[0153] Example 3 3-cyclopentyl-3-{4-[2-(phenylamino)thieno[3,2-d]pyrimidin-4-yl]-1H-pyrazol-1-yl}propionitrile T- 03 The synthetic route is as follows:
[0154]
[0155] Synthesis of compound 3-b
[0156] Under a nitrogen atmosphere, the suspension of cyanomethyltriphenylphosphine bromide (12g, 31.49mmol) in anhydrous tetrahydrofuran (100mL) was cooled to 0°C, and a 2.5M n-butyllithium n-hexane solution (13mL, 34.64 mmol). After continuing stirring at 0°C for 30 minutes, cyclopentylcarbaldehyde (3.1 g, 31.49 mmol) was added and stirred at room temperature for 1 hour. Add saturated ammonium chloride solution (50 mL) to quench the reaction, extract with ethyl acetate (100 mL×3), combine organic phases, wash with water (60 mL×3) and saturated brine (60 mL) successively, and dry over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com