Method for separating free nucleic acid from blood serum or blood plasma sample by using magnetic bead
A technology of free nucleic acid and magnetic beads, which is applied in the field of nucleic acid purification, can solve the problems of poor instrument universality, cumbersome steps, and increased costs, and achieve the effect of saving time and simplifying steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
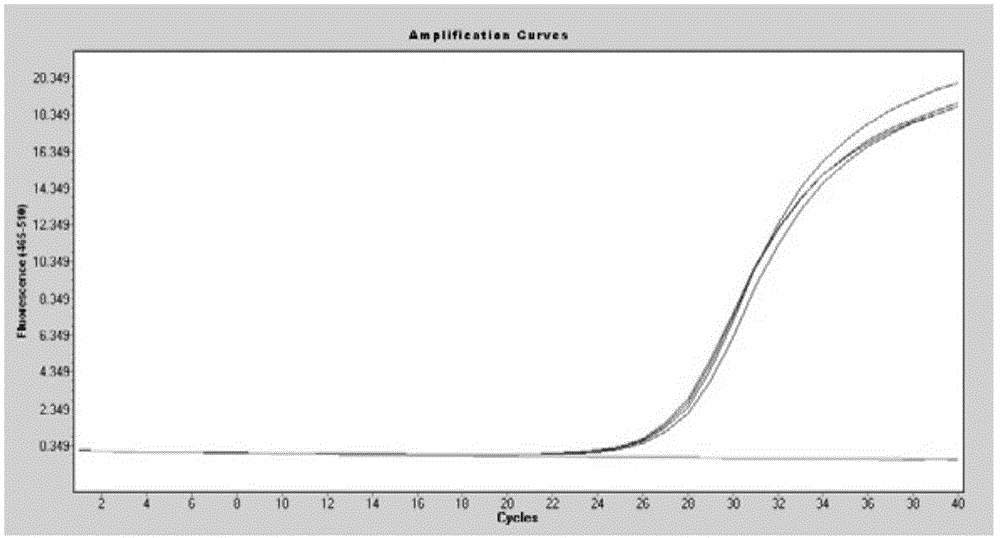
[0059] Example 1: The embodiment of extracting free nucleic acid from 200 microliters of serum samples
[0060] 1. Add 200 μl serum / plasma sample and 300 μl lysis buffer to a 1.5 ml nuclease-free centrifuge tube;
[0061] 2. Add 20 microliters of proteinase K and 15 microliters of magnetic bead suspension (shake well before use) to the above mixture, mix well, blow with a pipette or mix upside down for 10 minutes at room temperature;
[0062] 3. Place the centrifuge tube in step 2 on the magnetic stand, and magnetically adsorb for 1 minute, so that the magnetic beads are completely adsorbed on the wall of the centrifuge tube, and discard the supernatant;
[0063] 4. Remove the centrifuge tube in step 3 from the magnetic stand, add 500-600 microliters of the first washing buffer, and mix with a pipette or vortex. Place the centrifuge tube on the magnetic stand, magnetically adsorb for 1 minute, and discard the supernatant;
[0064] 5. Remove the centrifuge tube in step 4 from...
Embodiment 2
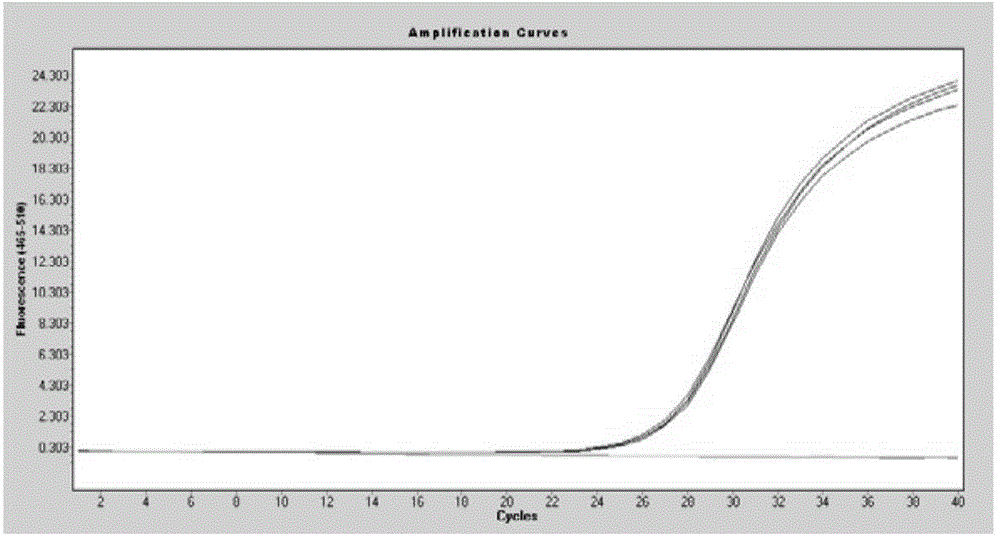
[0091] Example 2: The implementation of extracting free nucleic acid from 200 microliters of plasma samples:
[0092] 1. Add 200 microliters of plasma sample and 300 microliters of lysis buffer to a 5 milliliter nuclease-free centrifuge tube;
[0093] 2. Add 20 microliters of proteinase K and 15 microliters of magnetic bead suspension (shake well before use) to the above mixture, mix well, blow with a pipette or mix upside down for 10 minutes at room temperature;
[0094] 3. Place the centrifuge tube in step 2 on the magnetic stand, and magnetically adsorb for 1 minute, so that the magnetic beads are completely adsorbed on the wall of the centrifuge tube, and discard the supernatant.
[0095] 4. Remove the centrifuge tube in step 3 from the magnetic stand, add 500-600 microliters of the first washing buffer, and mix with a pipette or vortex. Place the centrifuge tube on the magnetic stand, magnetically adsorb for 1 minute, and discard the supernatant;
[0096] 5. Remove the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com