LSD1 inhibitors and application thereof
A technology of inhibitors and compounds, applied in the field of medicine, can solve problems such as few and no drugs on the market
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
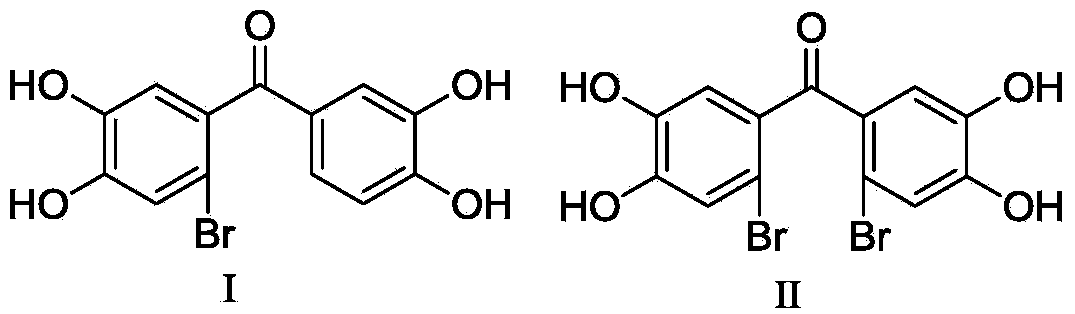
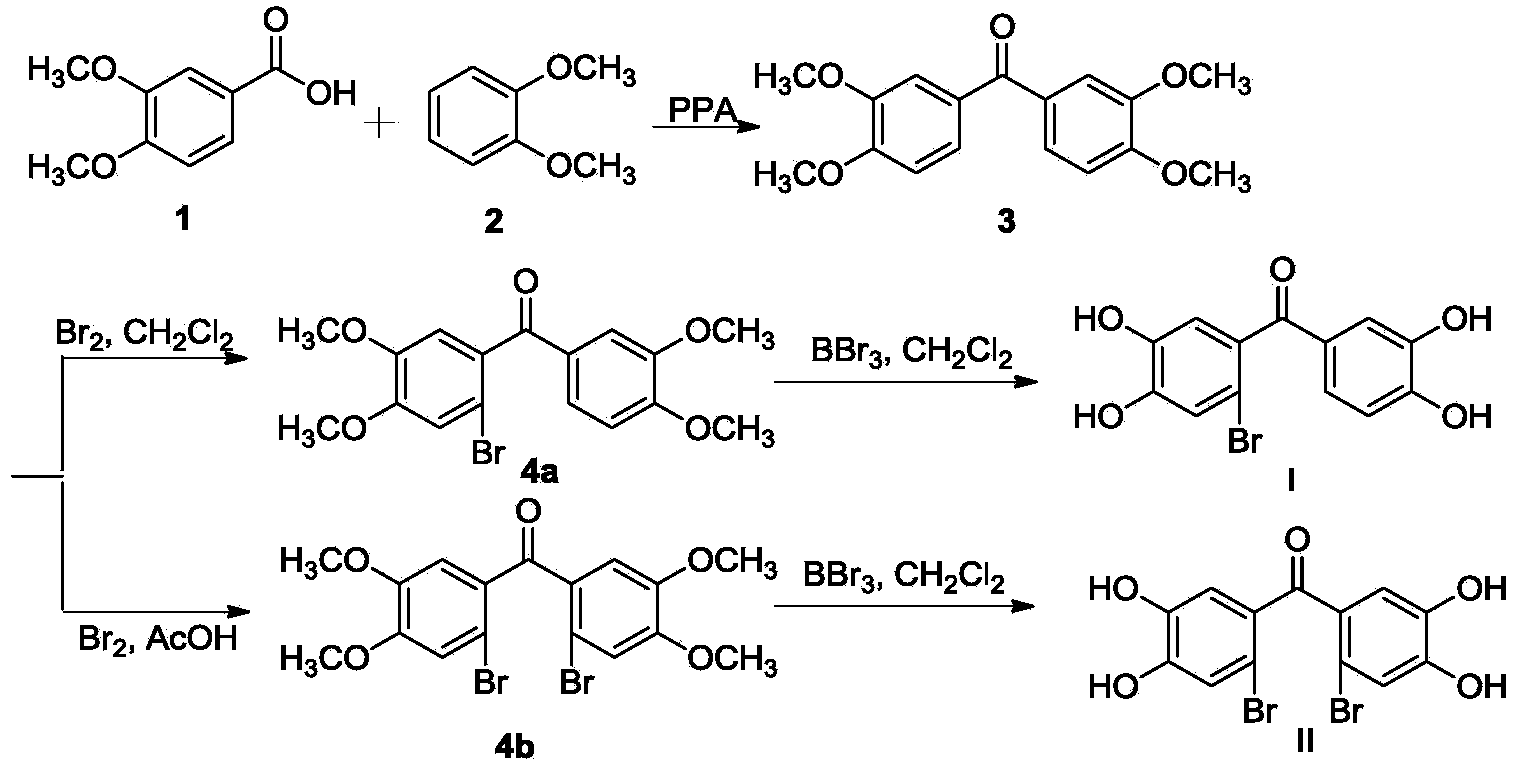
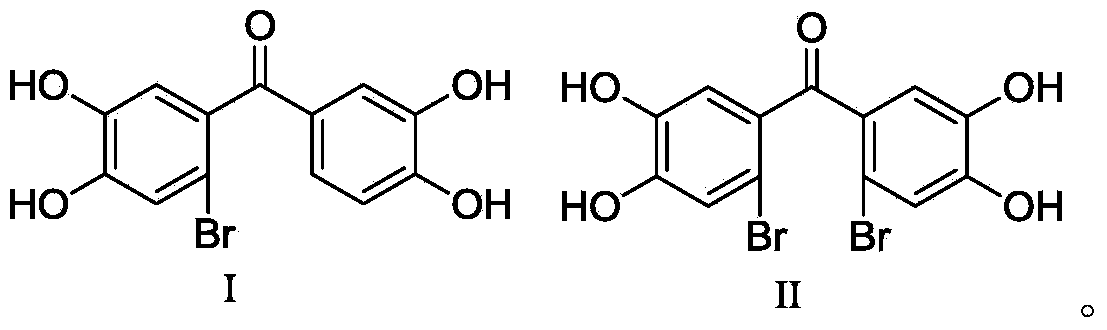
[0020] (3′,4′-Dihydroxy-phenyl)-(2-bromo-4,5-dihydroxy-phenyl)-methanone (I) and (2′-bromo-4′,5′-dihydroxy Preparation of phenyl)-(2-bromo-4,5-dihydroxyphenyl)-methanone (II).
[0021] The synthetic route of the compound is shown in the figure below:
[0022]
[0023] (1) After adding 22.08g (120mmol) of veratrolic acid, 16.92g (120mmol) of veratrole and 100g of polyphosphoric acid into a 500ml three-necked flask, the mixture was reacted under electric stirring at 80°C for 1 hour. The reaction mixture was cooled to 60° C. and 250 ml of ice water was added dropwise to the reactant within 30 min. At this time, a large amount of water-insoluble pink solid appeared in the reactant. After removing water by filtration, the obtained solid was dissolved in 100 ml of dichloromethane, and then the dichloromethane phase was washed 3 times with an equal volume of 3% sodium hydroxide solution and distilled water, respectively. After drying over anhydrous magnesium sulfate, it was conc...
Embodiment 2
[0029] Lysine-specific histone methylase 1 (LSD1) inhibitory activity assay
[0030] Human recombinant LSD1 / CoREST was expressed in Escherichia coli as an independent protein and co-purified using the method reported in the literature (Forneris F et al. Trends Biochem Sci, 2008, 33(4):181-189). Enzyme activity and inhibition experiments refer to methods reported in literature (Forneris F, et al. J. Biol. Chem. 282, 20070-20074), and a screening system was established. Compounds I and II were dissolved in a small amount of DMSO (the weight-to-volume ratio of compound to DMSO: 100mg: 1ml), diluted with buffer solution (50mM Hepes / NaOH with a pH of 7.5), and 2 μL was added to a 30 μL reaction system. Make the final concentrations of 100 μM, 10 μM, 1.0 μM, 0.1 μM, and 0.01 μM respectively, use Lys4 monomethylated histone H3 peptide as a substrate, and measure the fluorescence at 665 nm and 615 nm wavelengths with Lance detection reagent from Perkin Elmer Company The signal intens...
Embodiment 3
[0033] Preparation of compound (I) and (II) injection
[0034] Compounds (3′,4′-dihydroxy-phenyl)-(2-bromo-4,5-dihydroxy-phenyl)-methanone (I) and (2′-bromo-4′,5′-di Hydroxyphenyl)-(2-bromo-4,5-dihydroxyphenyl)-methanone (II) with a small amount of DMSO (weight ratio: 1:0.1-1:0.5, here is 1:0.4) After dissolving, add water for injection as usual (weight ratio is 1:20-1:200, here is 1:200), fine filter, potting and sterilizing to make injection solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com