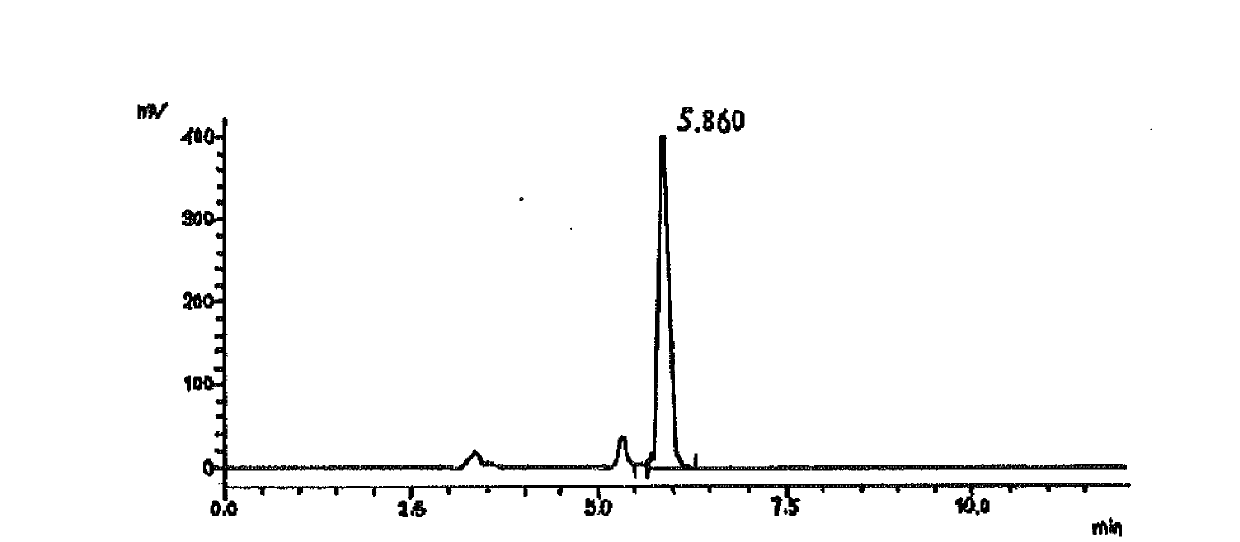
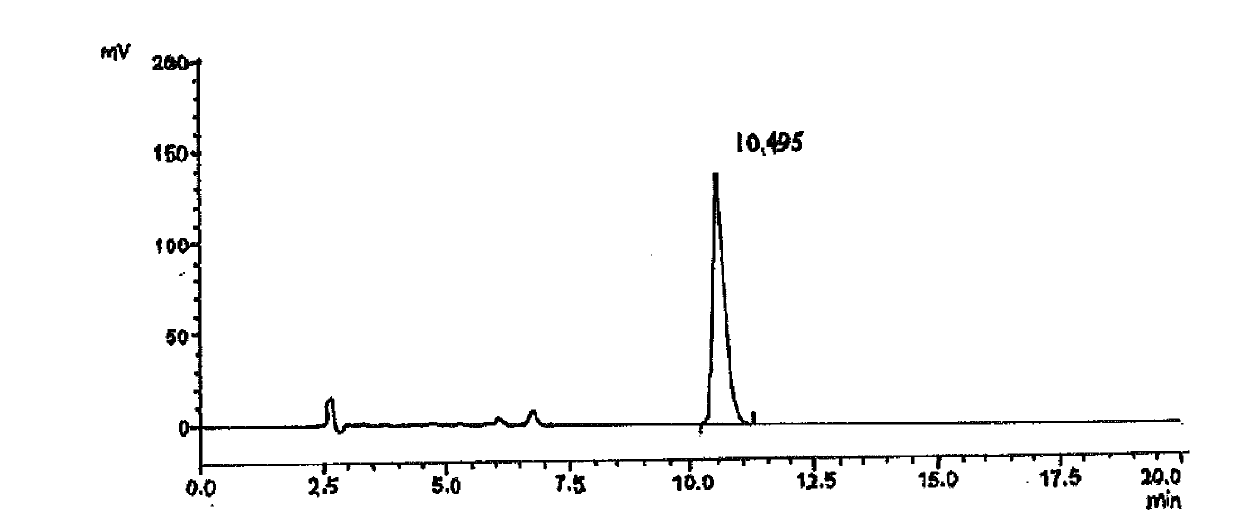
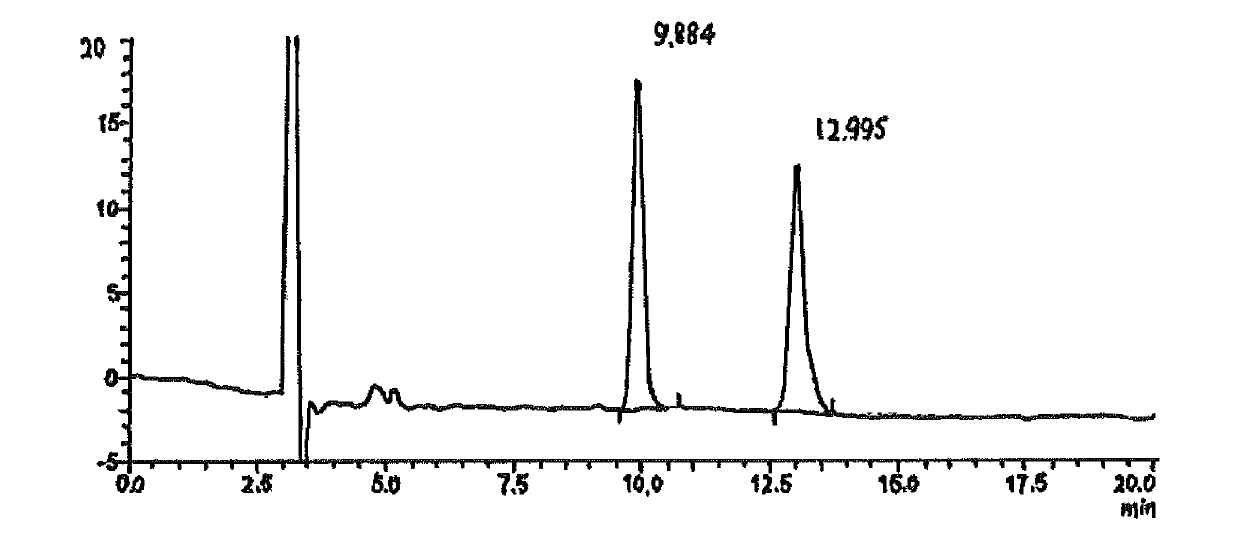
Saxagliptin intermediate, its salt, preparation method and application
An intermediate and inert gas technology, applied in the field of saxagliptin intermediates, can solve problems such as cumbersome operation, harsh reaction conditions, and difficult control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Preparation of ethyl 3-hydroxy-1-adamantanyl ketoate (compound B, R 1 group is ethyl)
[0087] Under the protection of nitrogen, add 50g (220mmol) of 3-hydroxy-1-adamantanyl ketoacid into the reaction flask, add it into 500ml of absolute ethanol, dissolve and clarify, lower the temperature to 0°C, and slowly add Equivalent of 26.56g of thionyl chloride, keep the temperature at 0-5°C, after the dropwise addition is completed, raise the temperature to reflux, react for 1 hour, evaporate the reaction solution to dryness under reduced pressure, add 200ml of toluene, and extract with 200ml of water , liquid separation, the toluene layer was washed with 100ml of aqueous sodium bicarbonate solution, 100ml of salt water, and separated, and the organic layer was washed twice with 50ml of salt water. After the liquid separation, the organic layer was dried and evaporated to dryness to obtain the product 3- Ethyl hydroxy-1-adamantanyl ketoate, white solid 50g, yield 90%.
Embodiment 2
[0089] Preparation of the compound of general structural formula 2 (R 1 group is ethyl)
[0090] Under the protection of nitrogen, add 50g (220mmol) of ethyl 3-hydroxy-1-adamantanyl ketone into the reaction flask, add it into 500ml of toluene, dissolve and clarify, then add 26.4g of R-phenylethylamine
[0091] (242mmol), add 0.5g of p-toluenesulfonic acid, install a water separator, heat to reflux to separate water, react for 24 hours, after the reaction is completed, evaporate to dryness under reduced pressure to obtain 70g of brown oil, with a yield of 99%.
Embodiment 3
[0093] Preparation of 1S-(3-hydroxyl-1-adamantyl)-(1R-phenyl-ethylamino)-acetic acid ethyl ester hydrochloride (structural formula 1, R 1 for ethyl)
[0094] Put 70g of the product prepared in Example 2 into the reaction flask, add 700ml of acetonitrile to dissolve and clarify, ice-bath to 5°C, add 11g of sodium borohydride in batches, maintain -5~0°C, slowly add 140g of acetic acid dropwise, and stir the reaction After 2 hours, the reaction is complete, evaporate the solvent to dryness under reduced pressure, add 200ml of ethyl acetate, extract with 200ml of water, use 6N aqueous sodium hydroxide solution to adjust pH=8-9, let stand to separate layers, and extract with 100ml of ethyl acetate For the aqueous phase, the combined organic layer was heated to 45°C, concentrated hydrochloric acid was added dropwise, and the pH was adjusted to 3-4. Granular crystals were precipitated after the dropwise addition, kept for 20 minutes, cooled to room temperature, stirred overnight, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com