Benzamide compound with antitumor activity as well as preparation method and application thereof
A technology of anti-tumor activity and benzamide, which is applied in the field of biomedicine, can solve the problems of uncontrollable side effects, inability to achieve therapeutic effects of chemical drugs, and drug resistance, and achieve cheap reagents, mild reaction conditions, and easy raw materials. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
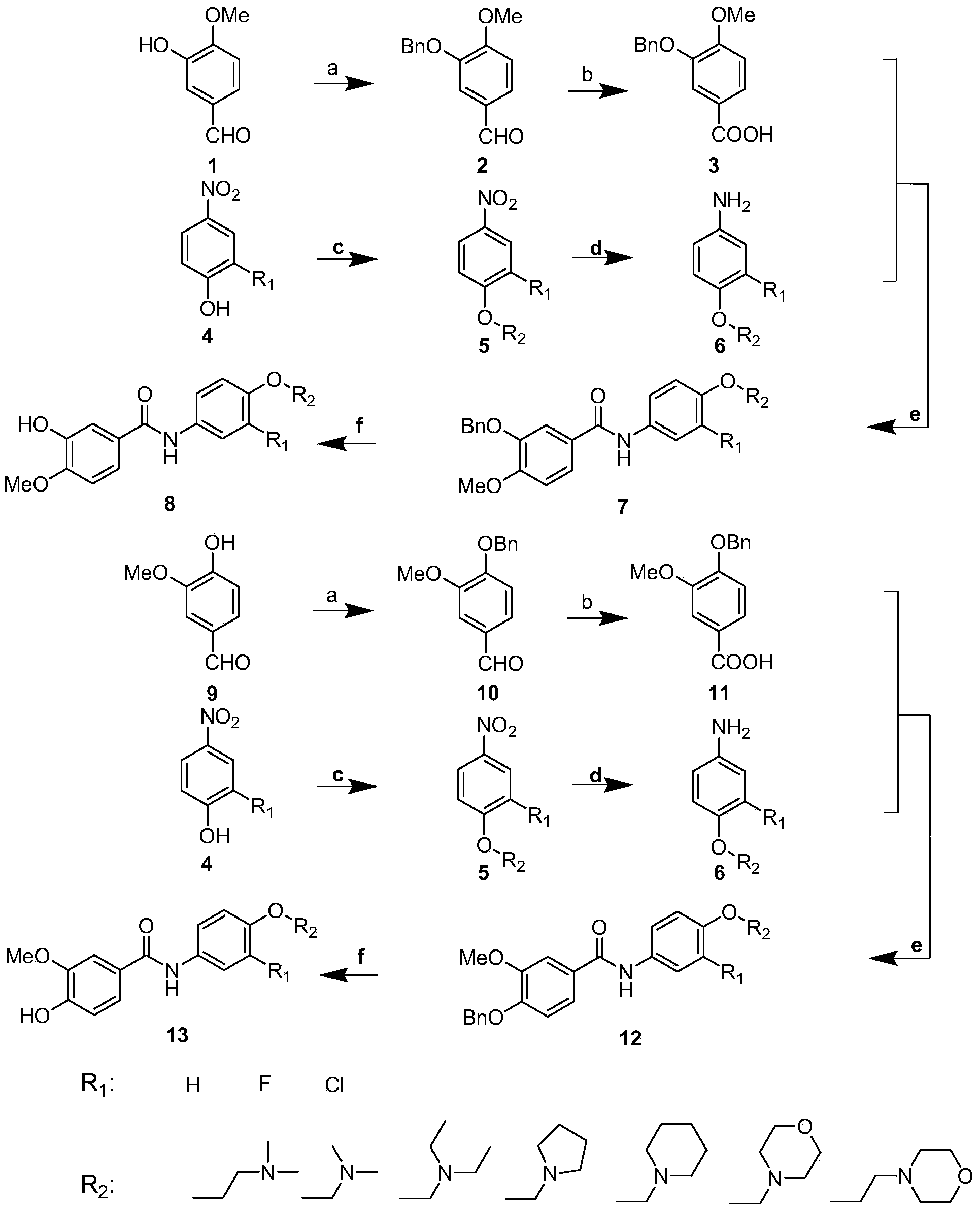
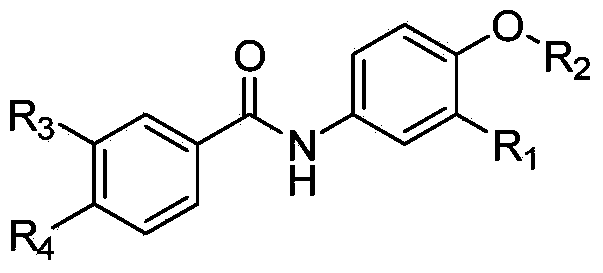
[0040] In the structural formula of this compound, R 1 is a hydrogen atom, R 2 It is an alkoxy group with 2 carbon atoms, and the end is substituted by a morpholinyl group, prepared by the following steps (see figure 1 ):
[0041] 1) 3-Hydroxy-4-methoxybenzaldehyde (1) Preparation of compound 3-benzyloxy-4-methoxybenzaldehyde (2) by benzylation reaction
[0042]Dissolve 4.56g (30mmol) of 3-hydroxy-4-methoxybenzaldehyde (1) in 120mL of absolute ethanol, add 12.44g (90mmol) of anhydrous potassium carbonate, stir at room temperature for ten minutes, add 5.20mL (45mmol) Benzyl chloride was heated to reflux for 5 hours. After the reaction is completed, cool to room temperature and filter, the filtrate is decompressed to recover ethanol, then add 140mL ethyl acetate for extraction, the organic phase is washed with dilute hydrochloric acid, saturated sodium bicarbonate solution, water, and saturated sodium chloride solution successively, and finally the organic phase is washed wit...
Embodiment 2
[0056] where R 1 is a fluorine atom, R 2 It is an alkoxy group with 3 carbon atoms, and the end is substituted by dimethylamino, prepared by the following steps
[0057] Steps 1) to 2) are the same as in Example 1, that is, the compound 3-benzyloxy-4-methoxybenzoic acid (3-benzyloxy-4-methoxybenzoic acid (3 ), and then with [3-(4-amino-2-fluorophenoxy) propyl] dimethylamine (6) to prepare N-{4-[3-(dimethylamino) propoxy] through condensation reaction -3-fluorophenyl}-3-benzyloxy-4-methoxybenzamide (7), the specific embodiment is:
[0058] Dissolve 0.98g (3.8mmol) of 3-benzyloxy-4-methoxybenzoic acid (3) in 100mL of anhydrous tetrahydrofuran, then add 1.46g (7.6mmol) of EDCI and 0.77g (5.7mmol) of HOBT in sequence, DMAP0.24g, triethylamine 5mL, continue to stir for 15min after the dropwise addition, then add dropwise 0.97g (4.6mmol) [3-(4-amino-2-fluorophenoxy)propyl]dimethyl 30 mL of anhydrous tetrahydrofuran solution of baseamine (6), and stirred at room temperature for 8...
Embodiment 3
[0064] where R 1 is a fluorine atom, R 2 It is an alkoxy group with 3 carbon atoms, and the end is substituted by a morpholinyl group, prepared by the following steps
[0065] Steps 1) to 2) are the same as in Example 1, that is, the compound 3-benzyloxy-4-methoxybenzoic acid (3-benzyloxy-4-methoxybenzoic acid (3 ), and then prepare N-[3-fluoro-4-(3-morpholine- 4-ylpropoxy)phenyl]-3-benzyloxy-4-methoxybenzamide (7), the specific embodiment is:
[0066] Dissolve 0.98g (3.8mmol) of 3-benzyloxy-4-methoxybenzoic acid (3) in 100mL of anhydrous tetrahydrofuran, then add 1.46g (7.6mmol) of EDCI and 0.77g (5.7mmol) of HOBT in sequence, DMAP0.24g, triethylamine 5mL, continue to stir for 15min after the dropwise addition, then add dropwise 1.16g (4.6mmol) [3-fluoro-4-(3-morpholin-4-ylpropoxy) to the reaction solution 30 mL of anhydrous tetrahydrofuran solution of phenyl]amine (6), stirred at room temperature for 8 hours after the dropwise addition was completed. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com