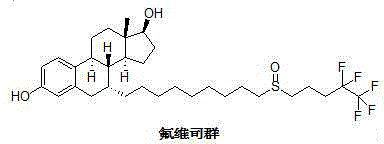
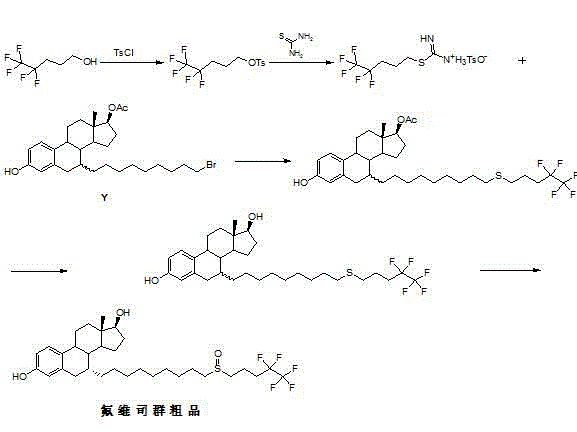
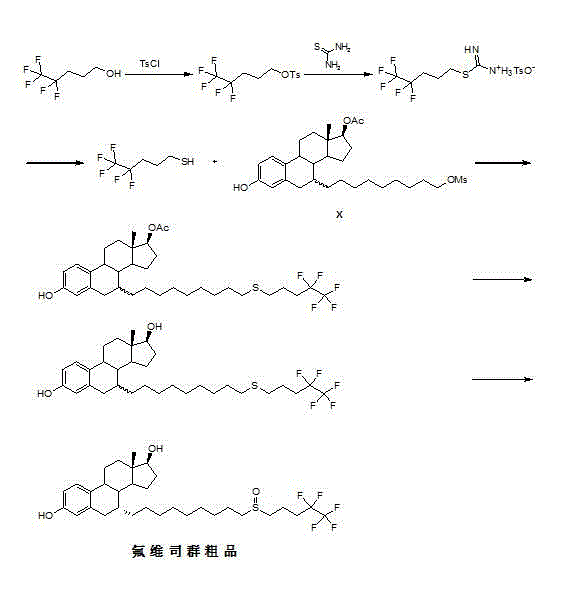
New fulvestrant synthesis method
A technology of fulvestrant and a synthesis method, applied in the field of pharmaceutical organic chemistry synthesis, can solve problems such as increasing difficulty, increasing project cost, and long route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of Intermediate X:
[0046](1) Heat and melt 1 kg of industrial-grade 1,9-nonanediol, wash it with water three times, and obtain 673 g of 1,9-nonanediol containing 0.057% of 1,8-octanediol;
[0047] (2) Add 1,9-nonanediol (1 kg) to toluene (20 L), add hydrobromic acid (40%, 2.5 L) under stirring, and heat to reflux (internal temperature 90-100°C) for 24 hrs. Stop heating, cool to 40°C, add ethyl acetate (4L) and cold water (4L), stir and separate layers, the organic phase is washed with saturated sodium bicarbonate aqueous solution and aqueous solution successively, dried with anhydrous sodium sulfate, and spin-dried to obtain 1.6 kg Add 4.8 L of n-hexane to the crude product, stir and heat to dissolve, cool and crystallize to obtain 1.1 kg 9-bromo-1-nonanol.
[0048] (3) Add 9-bromo-1-nonanol (1.1 kg), N,N-dimethylformamide (2.86 L), imidazole (363 g) into a 10 L reaction flask, add di Methyl tert-butylchlorosilane (772 g), the temperature is lower than 2...
Embodiment 2
[0055] (1) Add 4,4,5,5,5-pentafluoropentanol (10 g) and dichloromethane (100 mL) into the reaction flask, add triethylamine (11.3 g), dropwise under ice-cooling Methanesulfonyl chloride (6.72 g) was added dropwise, and returned to room temperature to react for 2 hours. Excess triethylamine and methanesulfonyl chloride were washed with water, dried over anhydrous sodium sulfate, and spin-dried to obtain 14.14 g of compound II.
[0056] (2) Add 4,4,5,5,5-pentafluoropentanol (10 g), toluene (80 mL), and hydrobromic acid (40%, 26 mL) into the reaction flask in sequence, heat to reflux, and react After 24 hours, it was cooled to room temperature, washed successively with aqueous sodium bicarbonate solution and water, dried over sodium sulfate, and used directly in the next reaction.
[0057] (3) Add 4,4,5,5,5-pentafluoropentanol (10 g) and 1,2-dichloromethane (80 mL) into the reaction flask in turn, add thionyl chloride ( 6.7 g), the dropwise addition was completed, heated to 30°...
Embodiment 3
[0059] (1) Dissolve compound II (20g) in N,N-dimethylformamide (200 mL), add thiourea (6.6g), heat to reflux overnight, cool to room temperature, and put it directly into the next reaction.
[0060] (2) Add the toluene solution of compound II to the reaction flask, add methanol (20 mL) and thiourea (3.84 g) in sequence, heat to reflux overnight, cool to room temperature, extract with water (100 mL), and use the aqueous phase directly for Next reaction.
[0061] (3) Add the 1,2-dichloromethane solution of compound II into the reaction flask, add methanol (20 mL) and thiourea (3.84 g) in sequence, heat to reflux overnight, cool to room temperature, and extract with water (100 mL) , and the aqueous phase was directly used for the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com