Method for preparing acetylpropionic acid by catalytically converting lignocelluloses
A lignocellulose, catalytic conversion technology, applied in the field of one-step catalytic conversion of lignocellulose to prepare levulinic acid, can solve the problems of high pollution energy consumption, long reaction time, environmental pollution, etc., to avoid equipment corrosion, simple synthesis method, The effect of overcoming circulatory difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
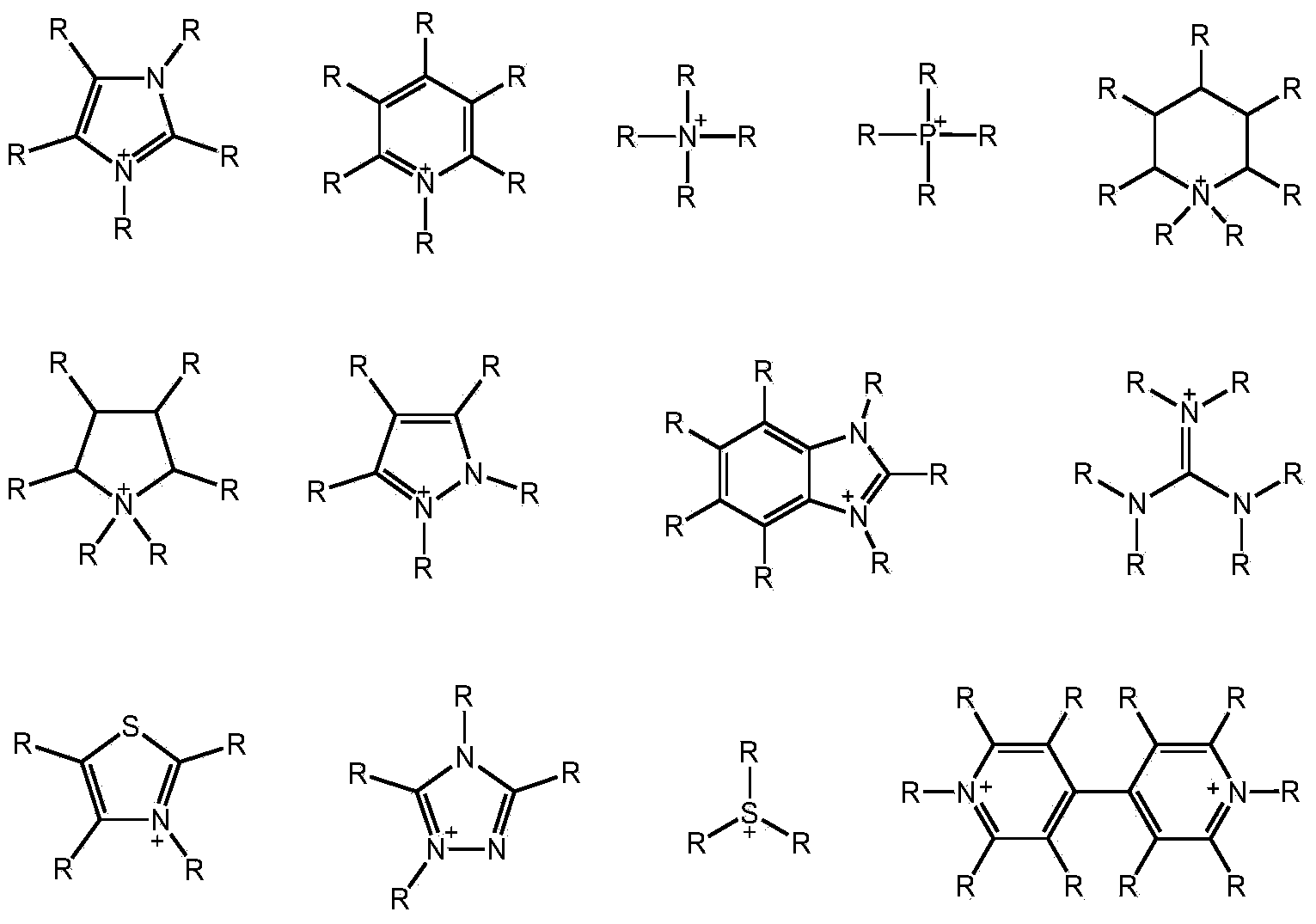
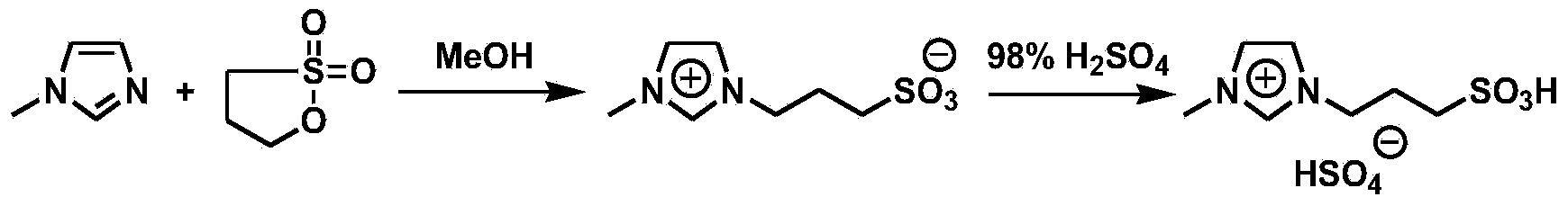
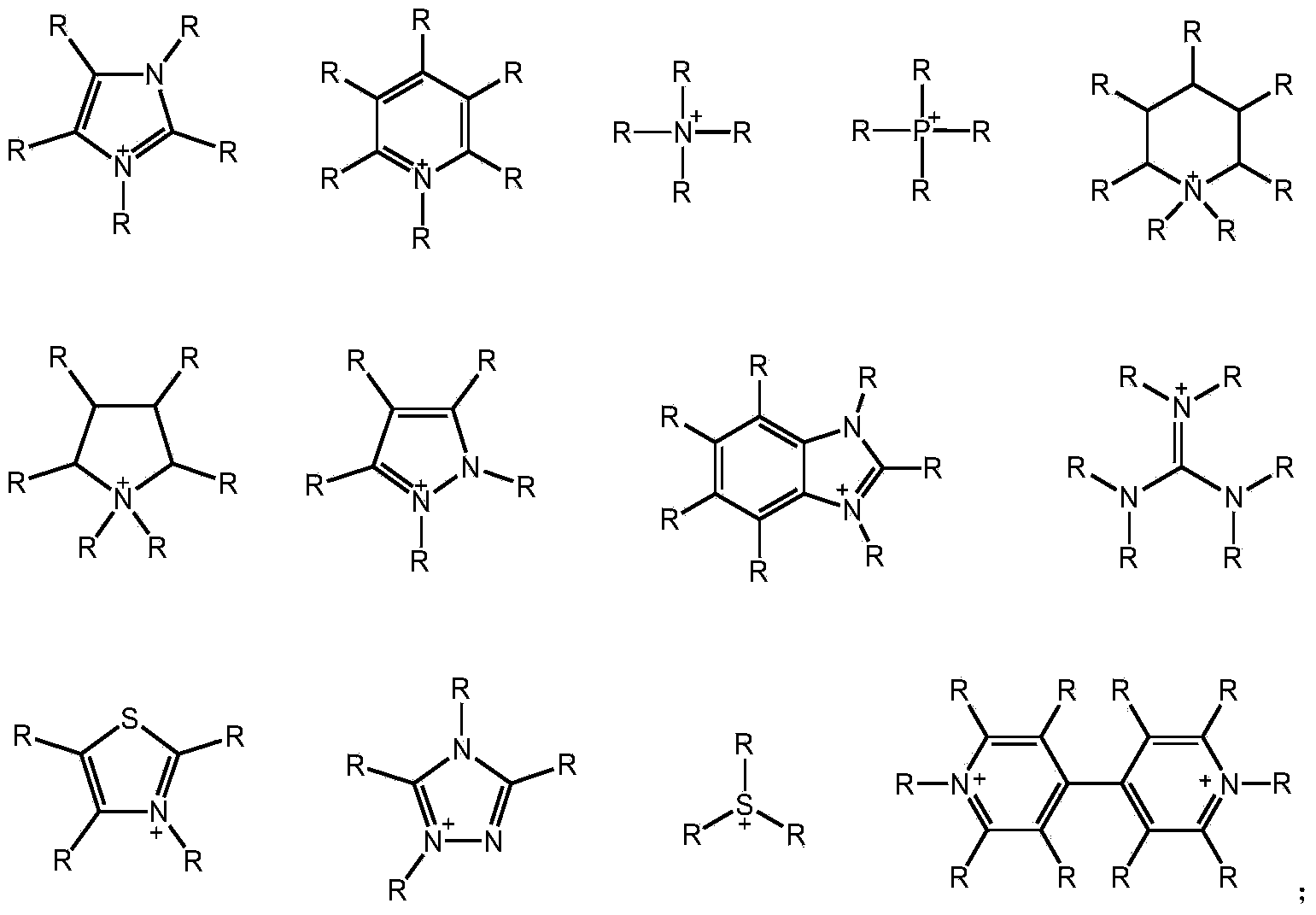
[0029] Lignocellulose (0.250g), 1-methyl-3-(3-sulfonate) propylimidazolium bisulfate ([C 3 SO 3 Hmim] HSO 4 ) ionic liquid (1.000g) and distilled water (2.000g) were mixed and reacted at 170°C for 30min. The reaction solution was extracted three times with 30 mL of dichloromethane, and the extract was concentrated to obtain levulinic acid with a yield of 3%. The ionic liquid could be recycled.
Embodiment 2
[0031] Lignocellulose (0.250g), 1-methyl-3-(3-sulfonate) propylimidazolium bisulfate ([C 3 SO 3 Hmim] HSO 4 ) ionic liquid (1.000g) and distilled water (2.000g) were mixed and reacted at 170°C for 60min. The reaction solution was extracted three times with 30 mL of dichloromethane, and the extract was concentrated to obtain levulinic acid with a yield of 12%. The ionic liquid could be recycled.
Embodiment 3
[0033] Lignocellulose (0.250g), 1-methyl-3-(3-sulfonate) propylimidazolium bisulfate ([C 3 SO 3 Hmim] HSO 4 ) ionic liquid (1.000g) and distilled water (2.000g) were mixed and reacted at 170°C for 90min. The reaction solution was extracted three times with 30 mL of dichloromethane, and the extract was concentrated to obtain levulinic acid with a yield of 16%. The ionic liquid could be recycled.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com