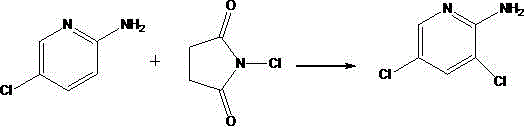
Synthetic method of 2-amino-3,5-dichloropyridine
A dichloropyridine and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of many by-products, high price, low product yield, etc., and achieve the effects of moderate reaction conditions, easy control of the reaction, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] In a 100ml round-bottom single-necked flask, add 45ml of a mixed solvent with a volume ratio of DMF and methanol of 1:1.5, insert a thermometer, start a magnetic stirrer, and add 12.8g of 2-amino-5-chloropyridine, add N- 6.75 g of chlorosuccinimide was reacted under stirring at 15° C. for 5 hours. TLC and GC confirmed the completion of the reaction. The solvent was removed by rotary evaporation to obtain the crude product, which was separated by silica gel column chromatography to obtain the pure product 2-amino-3,5-dichloropyridine. After drying, the calculated yield was 53.1%, and the purity was 96.28% (GC). NMR analysis: 1HMR (CDCl3) 400MHz: δ7.94 (s, 1H); δ7.51 (s, 1H); δ4.93 (bs, 2H). Melting point 80°C-84°C (79°C-83°C in literature).
Embodiment 2
[0022] Add 700ml of a mixed solvent of DMF and methanol at a volume ratio of 1.5:1 to a 2L round-bottom single-necked flask, insert a thermometer to start a magnetic stirrer, and add 169.7g of 2-amino-5-chloropyridine, N-chloro 264.4 g of succinimide was reacted with stirring at 0° C. for 8.5 hours. TLC and GC confirmed the completion of the reaction. The solvent was removed by rotary evaporation to obtain a crude product, and the pure product 2-amino-3,5-dichloropyridine was obtained by ethyl acetate. After drying, the calculated yield was 55.5%, and the purity was 98.50% (GC). Melting point 81°C-84°C (79°C-83°C in literature).
[0023]
Embodiment 3
[0025] Add 5500ml of a mixed solvent of DMF and methanol at a volume ratio of 2.5:1 to a 10L round-bottomed three-neck flask, insert a thermometer and install a condensation reflux device, start a magnetic stirrer, and add 2560.8g of 2-amino-5-chloro Pyridine, N-chlorosuccinimide 6118.4g, stirred and reacted at 45°C for 2.5 hours. TLC and GC confirmed the completion of the reaction. The solvent was removed by rotary evaporation to obtain a crude product, which was recrystallized from ethanol to obtain the pure product 2-amino-3,5-dichloropyridine. After drying, the calculated yield was 70.5%, and the purity was 98.20% (GC). Melting point 80°C-84°C (79°C-83°C in literature).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com