Extraction method for escherichia coli pilus antigen used for preparing yolk antibody, and method for preparing yolk antibody
A technology of Escherichia coli and extraction method, which is applied in the preparation of egg yolk antibody and the field of improved Escherichia coli fimbriae extraction, can solve the problems of toxicity of host cells, easy damage of bacterial cell walls, endotoxin shock and disseminated intravascular coagulation. , to achieve the effect of good antigenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
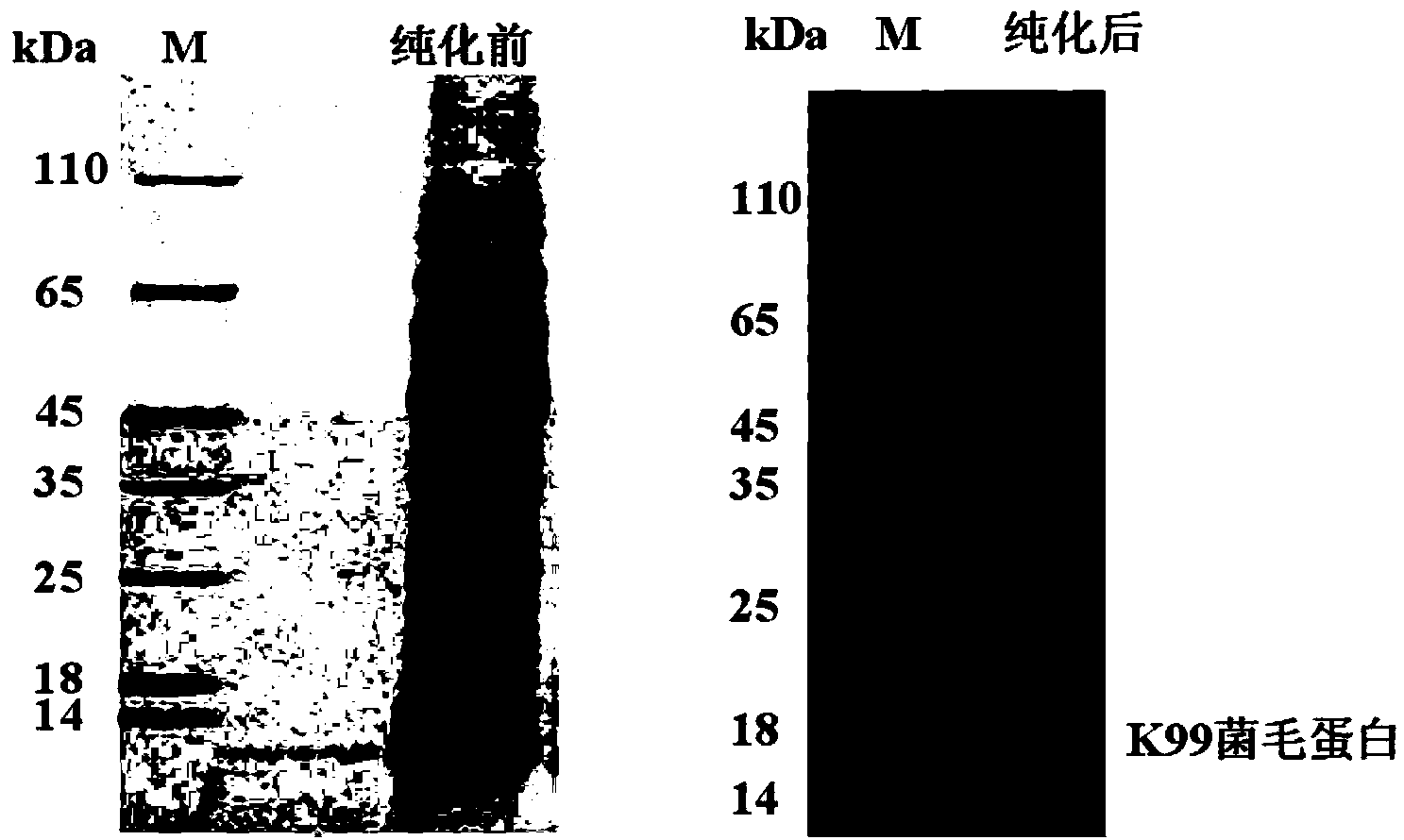
[0029] [Example 1] Enterotoxin Escherichia coli K99 pili extraction
[0030] Primary Seed Preparation
[0031] Prepare 100mL modified Minca culture based on a 500mL Erlenmeyer flask, and sterilize at 116°C for 30min. After cooling, inoculate K99 with an inoculum size of 2‰, culture at 37°C for 12-18h, and rotate at 200rpm.
[0032] Secondary Seed Preparation
[0033] Prepare two 500mL modified Minca cultures based on two 2000mL Erlenmeyer flasks, and sterilize at 116°C for 30min. After cooling, inoculate K99 primary seed solution with an inoculum size of 2‰, cultivate at 37°C for 12-18h, and rotate at 200rpm.
[0034] 15L Fermentation Tank High Density Fermentation
[0035] Prepare 15L of modified Minca culture-based fermenter, add an appropriate amount of antifoaming agent, and sterilize at 116°C for 30 minutes. After the sterilization is completed, when the culture temperature in the tank is 37°C, keep it for 12-24 hours for sterility test. After passing the sterility ...
Embodiment 2
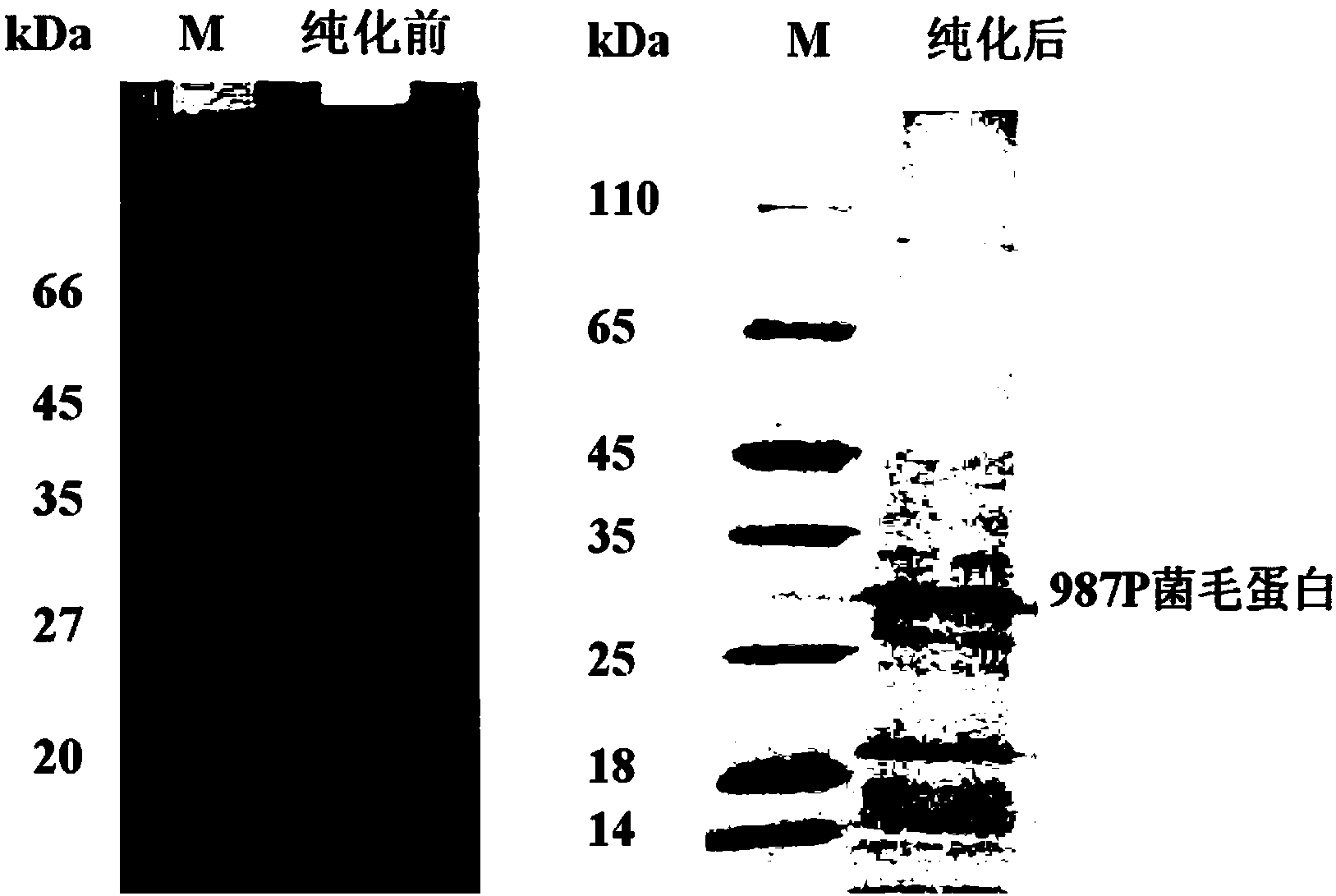
[0040] [Example 2] Enterotoxin Escherichia coli 987P pili extraction
[0041] The fermentation method of 987P is the same as that of K99. After the bacteria are collected, the pili protein is extracted by the following method:
[0042] 1. Weigh 100-150g of 987P fermented cells with a wet weight, add PBS (containing 2M urea) to dissolve (the volume ratio of cells to PBS is 1:10), and stir well with a stirrer. The fully dissolved bacteria were homogenized 5 times by a pressure breaker. The homogenate was processed by centrifugation at 8000rpm for 30min to remove the precipitate. The supernatant was then centrifuged at 18000rpm / 30min to remove the precipitate. The final supernatant was purified by 4FF chromatography column.
[0043]2. Equilibrate 2-3 volumes of 4FF agarose gel column with PBS (containing 2M urea) at a flow rate of 8ml / min, and turn on the UV detector at the same time. After the baseline is stable, measure 100ml of the crude pili extract, start loading the sam...
Embodiment 3
[0044] [Example 3] Removal of endotoxin
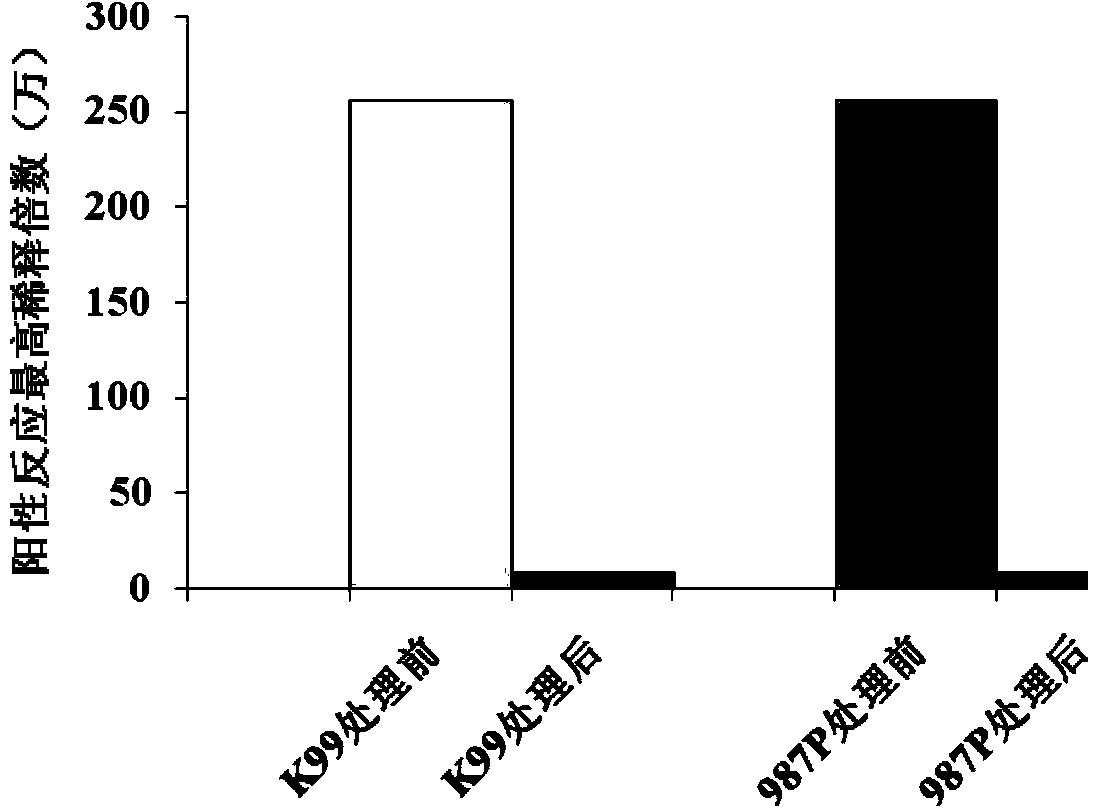
[0045] The sample solution obtained by the above method was added into acetone (under ice bath condition) at a volume ratio of 1:1, stirred for 10 min, centrifuged at 10,000 rpm for 20 min to remove the supernatant. Collect the precipitate, add PBS and acetone at a volume ratio of 1:9, stir in an ice bath for 10 min, and centrifuge at 10,000 rpm to discard the supernatant. After the acetone volatilized completely, the precipitate was redissolved in PBS to obtain the pili antigen. The change of endotoxin content was determined by the gel method limulus reagent, image 3 It can be seen that after the acetone treatment, the highest dilution factor at which the bacterial endotoxin reacted positively with the Limulus reagent decreased significantly, that is, the endotoxin content was significantly reduced, and no adverse reactions occurred after immunization of laying hens, and the obtained antigen can be used for immunization of laying he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com