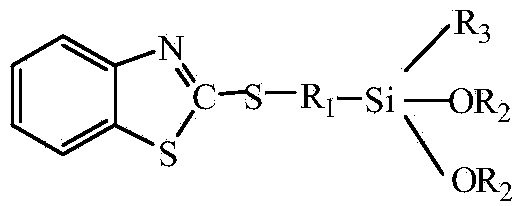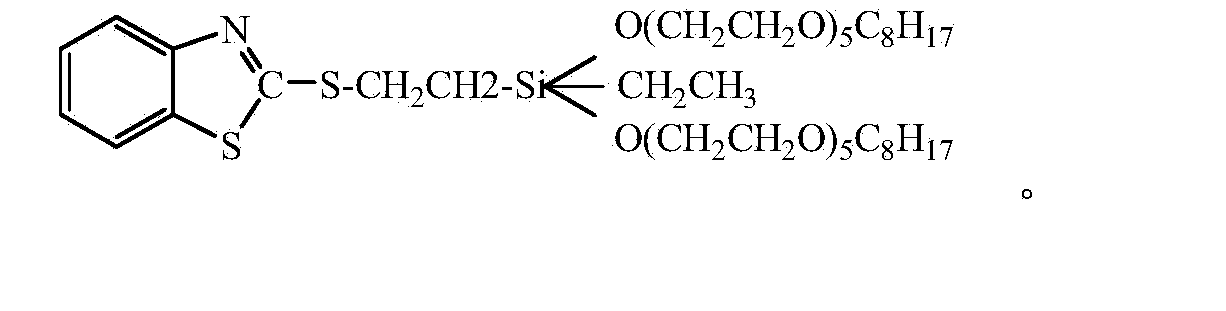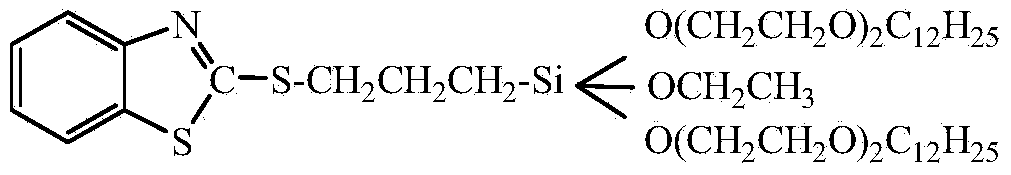Benzothiazole thiol silane prepared from 2-thiol benzothiazole and chloropropyl triethoxysilane
A technology of chloropropyltriethoxysilane and benzothiazolemercaptosilane, which is applied in the field of rubber additives, can solve the problems of polluting the processing workshop environment, physical damage to workers, and inability to remove methoxy and ethoxy groups. Achieve long scorch time, fast vulcanization speed, and improve dynamic mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] A kind of low volatility benzothiazole mercaptosilane, wherein, the molecular structural formula of silane is:
[0022]
[0023] The preparation method of above-mentioned silane is:
[0024] Step a: Weigh 0.1mol (28.86g) γ-chloropropyltriethoxysilane and 0.2mol (54.8g) polyether HO-(C 2 h 4 -O) 2 -C 12 h 25 Place in a three-necked flask, add 0.5g of zinc naphthenate, react at 70°C for 2 hours, and remove ethanol in a vacuum for one hour to obtain polyether-modified chloropropylsilane (ie, semi-finished silane);
[0025] Step b: Weigh 0.1mol (16.7g) of 2-mercaptobenzothiazole and 250g of toluene into a three-neck round bottom flask, add 0.1mol (5.4g) of sodium methoxide, stir well, and heat the mixture to 120°C, and from one side of the three-necked flask at a distance of 0.1m 3 Nitrogen was introduced at a speed of 1 / h, and then 75.3g of polyether modified chloropropylsilane was added to the above solution, and then reacted at 120°C for 10 hours under the prote...
Embodiment 2
[0040] A kind of low volatility benzothiazole mercaptosilane, wherein, the molecular structural formula of silane is:
[0041]
[0042] The preparation method of above-mentioned silane is:
[0043] Step a: Weigh 0.1mol (17.1g) γ-chloromethyltrimethoxysilane and 0.2mol (81.2g) polyether HO-(C 2 h 4 -O) 5 -C 12 h 25 Place in a three-necked flask, add 0.5g of sodium ethoxide, react at 165°C for 7 hours, and remove methanol in a vacuum for one hour to obtain polyether-modified chloropropylsilane (ie semi-finished silane);
[0044]Step b: Weigh 0.1mol (16.7g) of 2-mercaptobenzothiazole and 250g of toluene into a three-neck round bottom flask, add 0.1mol (5.4g) of sodium methoxide, stir well, and heat the mixture to 120°C, then add 81.4g of the above-mentioned polyether-modified chloropropylsilane to the above-mentioned solution, and then react at 50°C for 24 hours under the protection of nitrogen, filter the obtained product, wash, and remove the low-boiling fraction by dis...
Embodiment 3
[0059] A kind of low volatility benzothiazole mercaptosilane, wherein, the molecular structural formula of silane is:
[0060]
[0061] The preparation method of above-mentioned silane is:
[0062] Step a: take by weighing 5mol (862.5) gamma-chloropropyltrimethoxysilane and 10mol (508g) polyether HO-(C 2 h 4 -O) 7 -C 13 h 27 Put it in a reaction kettle, add 50g of butyl aluminate, and react under 0.09Mpa vacuum at 180°C for 10 hours to obtain polyether-modified chloropropylsilane (ie, semi-finished silane);
[0063] Step b: Weigh 5mol (835g) of 2-mercaptobenzothiazole and 10kg of acetone, place in a stainless steel container, add 7.5mol (300g) of sodium hydroxide, heat to 80°C, stir evenly, and blow in nitrogen , and then added to the above solution, and then reacted at 80°C for 6 hours under the protection of nitrogen, the obtained product, the sediment was filtered, and the low boiling point fraction was removed by vacuum distillation to obtain a brownish red liquid ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com