Chemically modified ursolic acid and its preparation method and use
A technology of chemical and chemical modification of ursolic acid, which is applied in the field of drug synthesis, can solve the problem of low inhibitory activity of α-glucosidase, and achieve the effects of wide application range, high synthesis yield and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 13
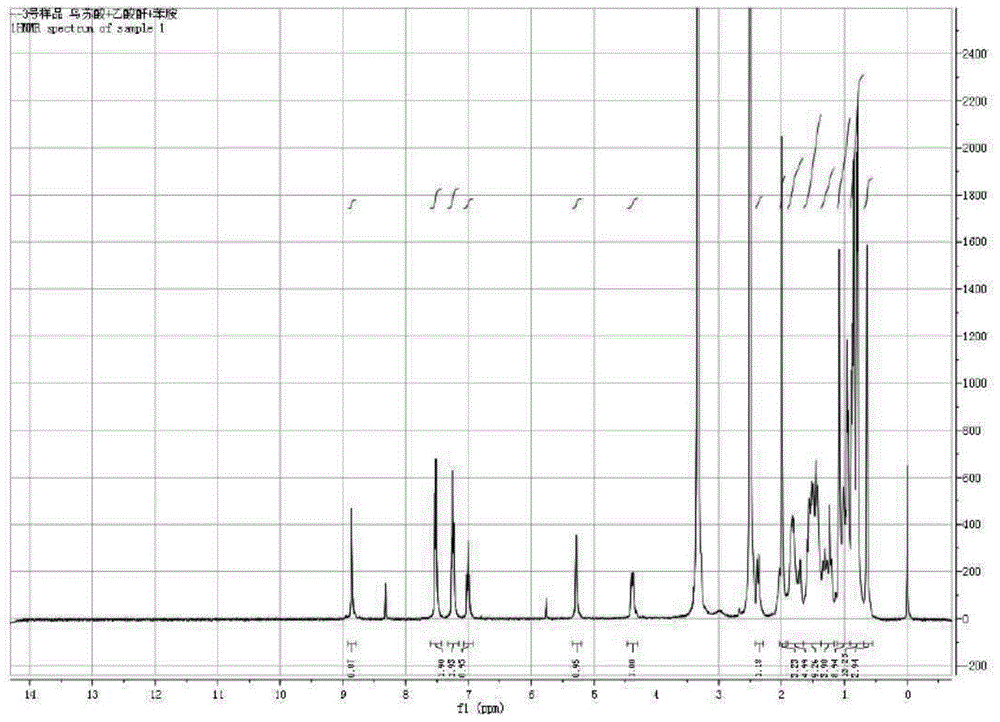
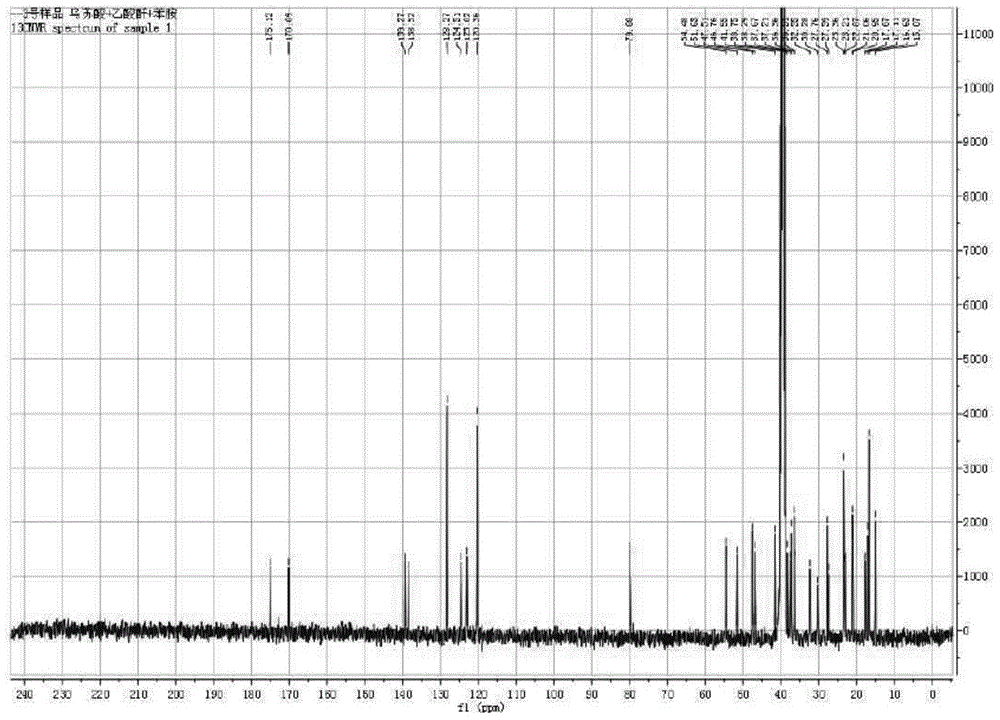
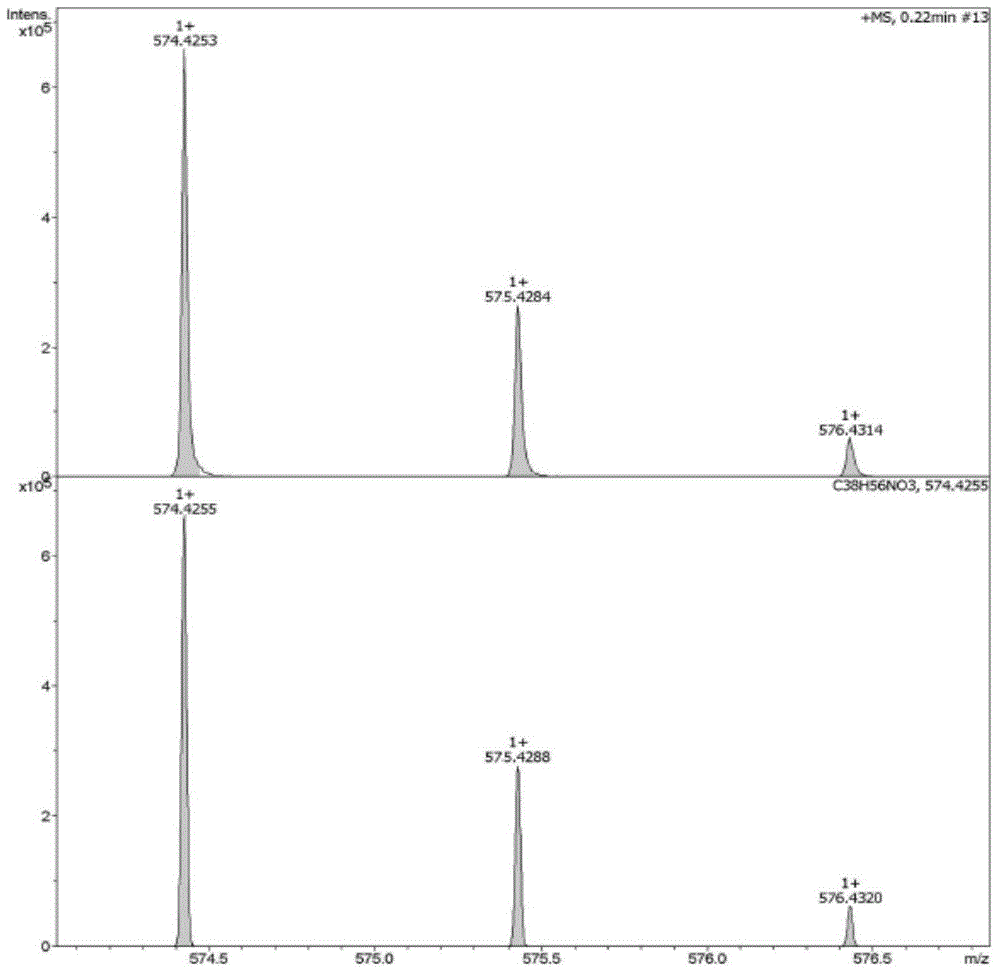
[0048]Example 13 Preparation of β-acetoxy-ursane-12-ene-28-carboxylic acid (compound 2):
[0049] First weigh 6mmol of ursolic acid (UA) into a 125mL round-bottomed flask containing magnets, then measure 75mL of dry pyridine into the flask, stir for about 10min at room temperature until it is completely dissolved, then add 60mg of 4-dimethyl Aminopyridine was stirred and dissolved, and then 24mmol (four times the amount) of acetic anhydride was slowly added dropwise, and reacted for 12 hours at room temperature, and the end point of the reaction was detected by TLC (developing agent: petroleum ether: ethyl acetate (volume ratio) = 5 :1, developer: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85:10:5:0.5). After the reaction was completed, pyridine was distilled off under reduced pressure at 55° C., and then the reaction system was dispersed with 100 mL of distilled water, suction filtered, and repeated three times to collect the solid layer....
Embodiment 23
[0050] Example 23 Preparation of β-propionyloxy-ursane-12-ene-28-carboxylic acid (compound 3):
[0051] First weigh 6mmol of ursolic acid (UA) into a 125mL round-bottomed flask containing magnets, then measure 75mL of dry pyridine into the flask, stir at room temperature for about 10min until it is completely dissolved, then add 60mg of 4-dimethylamino Stir to dissolve pyridine, then slowly add 24 mmol (four times the amount) of propionic anhydride dropwise, react at room temperature for 12 hours, and detect the end of the reaction with TLC (developing agent: petroleum ether: ethyl acetate (volume ratio) = 5:1 , Chromogenic agent: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85:10:5:0.5), the reaction is completed, at 55 ° C, evaporate pyridine under reduced pressure, and then disperse the reaction system with 100mL distilled water, Suction filtration was repeated three times to collect the solid layer. Disperse the solid with 100 mL of wat...
Embodiment 33
[0052] Example 33 Preparation of β-butyryloxy-ursane-12-ene-28-carboxylic acid (compound 4):
[0053] First weigh 6mmol of ursolic acid (UA) into a 125mL round-bottomed flask containing magnets, then measure 75mL of dry pyridine into the flask, stir at room temperature for about 10min until it is completely dissolved, then add 60mg of 4-dimethylamino Stir to dissolve pyridine, then slowly add 24 mmol (four times the amount) of butyric anhydride dropwise, react at room temperature for 12 hours, and detect the end of the reaction with TLC (developing agent: petroleum ether: ethyl acetate (volume ratio) = 5:1 , Chromogenic agent: methanol: acetic acid: concentrated sulfuric acid: anisaldehyde (volume ratio) = 85:10:5:0.5), the reaction is completed, at 55 ° C, evaporate pyridine under reduced pressure, and then disperse the reaction system with 100mL distilled water, Suction filtration was repeated three times to collect the solid layer. Disperse the solid with 100 mL of water a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com