Potassium aspartate tablet and preparation method thereof
A technology for potassium aspartate tablets and potassium aspartate tablets is applied in the directions of pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Poor moisture absorption and sticking of materials, etc., to achieve the effect of reducing hygroscopicity, reducing hygroscopicity and improving slivers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Prescription composition:
[0038]
[0039] Preparation:
[0040] (1) Binder configuration: weigh 30g of stearic acid, add it to 210g of ethanol (95%), heat to dissolve the stearic acid, and use it as a binder;
[0041] (2) Granulation: Weigh potassium aspartate and microcrystalline cellulose and mix evenly, add binder, stir evenly, and granulate with 18-mesh nylon screen; dry in a hot air circulation oven at 60°C for 4 hours; granules, add talcum powder and mix well;
[0042] (3) Tablet pressing: Use a rotary tablet press machine to press the tablet, the weight of the tablet is about 0.5g;
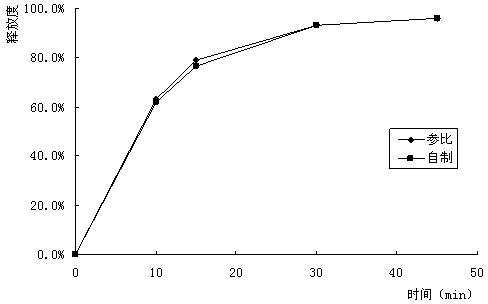
[0043] (4) Coating: Opadry (trade name, manufactured by Colorcon) was used for coating. Based on the plain tablet, the weight of the stomach-soluble moisture-proof coating increased by 3%.
[0044] In this example:
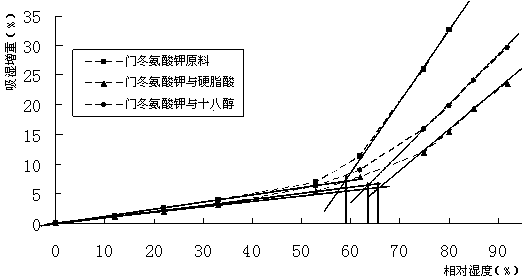
[0045] (1) The dosage of stearic acid in the tablet core formulation is 6%. Stearic acid is a hydrophobic material. After mixing with potassium aspartate, the s...
Embodiment 2
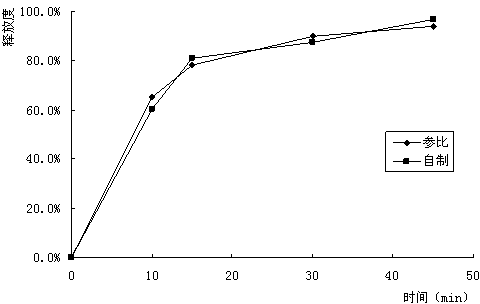
[0051] According to the method described in embodiment 1, utilize following prescription composition to prepare potassium aspartate tablet. Results Based on the plain tablet, the weight gain of the stomach-soluble moisture-proof coating was about 3%.
[0052] Prescription composition:
[0053]
Embodiment 3
[0055] According to the method described in embodiment 1, utilize following prescription composition to prepare potassium aspartate tablet. Results Based on the plain tablet, the weight gain of the stomach-soluble moisture-proof coating was about 3%.
[0056] Prescription composition:
[0057]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com