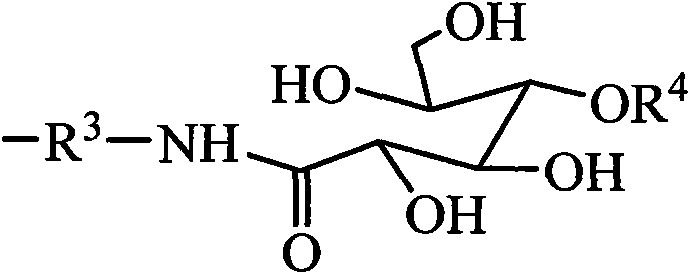
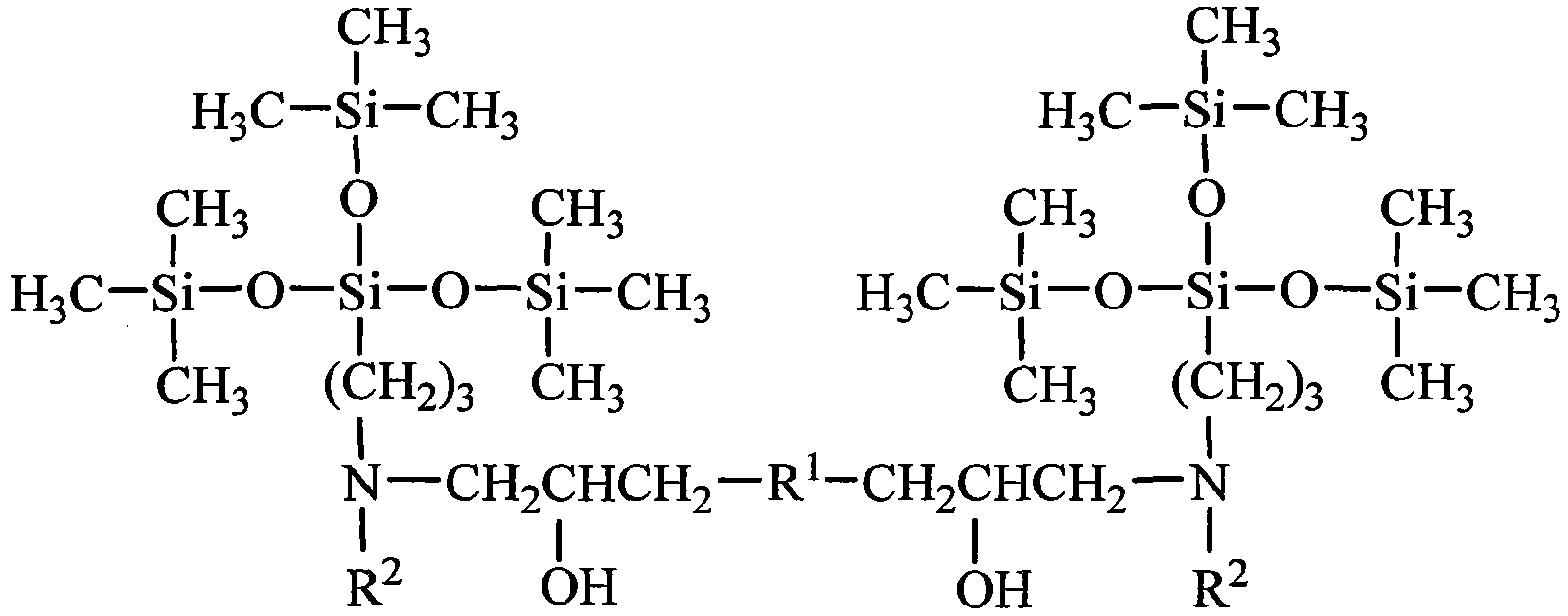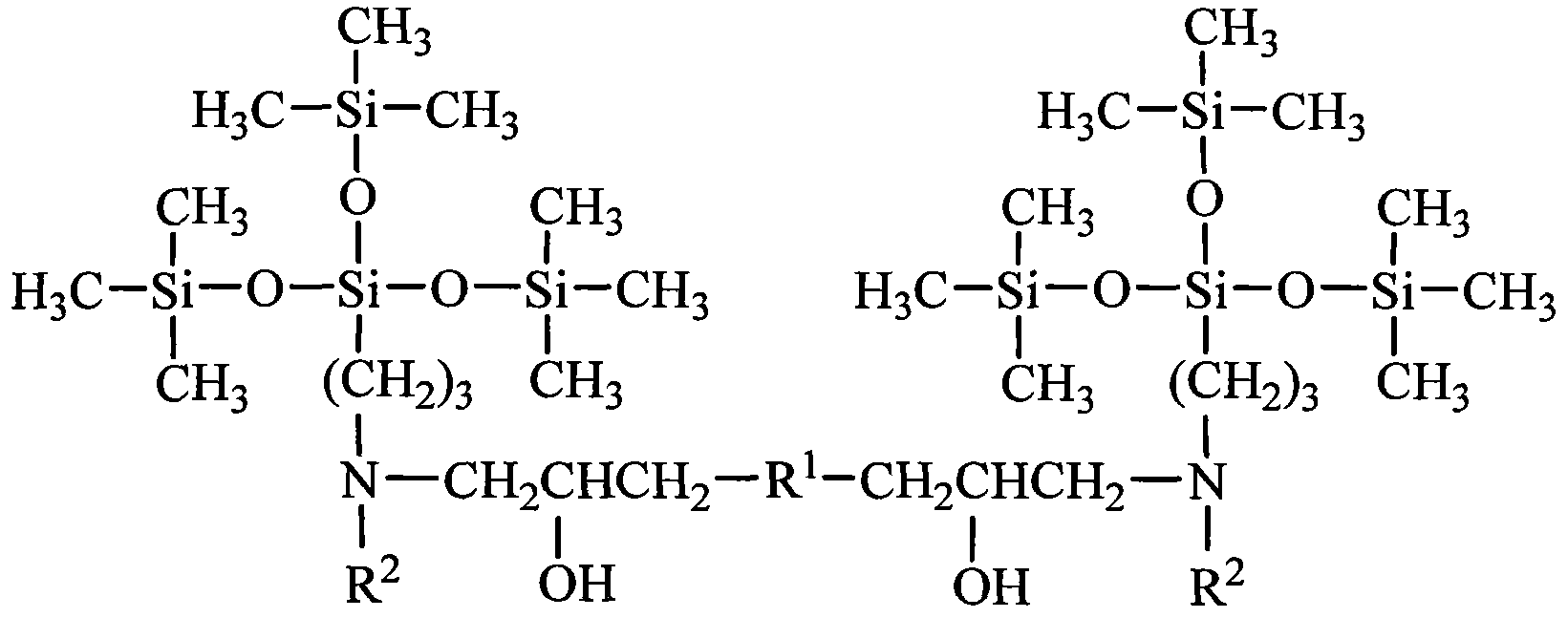Tetrasiloxane dimeric surfactant containing sugar acylamino and preparation method
A technology containing sugar amide group and tetrasiloxane, which is applied in the field of organosilicon compound preparation, can solve problems such as affecting the use performance and losing surface activity, and achieves the effect of good adsorption performance and difficult hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 1.62kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.006kg of concentrated sulfuric acid into reaction kettle 1, react at a temperature of 40°C for 4 hours, add sodium hydroxide to deactivate the catalyst, and filter The solid was removed and distilled under reduced pressure to obtain chloropropyltetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 0.74kg of 1,3-propylenediamine into the reaction kettle 2, heat to 60°C, and react for 4 hours. - Aminopropyl-γ-aminopropyltetrasiloxane. Add 4.10 kg of N-β-aminopropyl-γ-aminopropyltetrasiloxane and 1.78 kg of gluconolactone into reaction kettle 3, use methanol as solvent, heat to 60° C., and react for 8 hours. Add 0.87 kg of ethylene glycol diglycidyl ether again, and continue to react for 12 hours. The solvent methanol was distilled off to obtain a tetrasiloxane gemini surfactant containing sugar amide groups. It is measured that it does not hydrolyze within 6 months in water.
Embodiment 2
[0035] Add 8.12kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.10kg of acid clay to reaction kettle 1, react at a temperature of 50°C for 4 hours, remove the catalyst by filtration, and distill under reduced pressure to obtain chloropropane Tetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 2.64kg of 1,4-butanediamine into the reaction kettle 2, heat to 80°C, and react for 3 hours. - Aminobutyl-γ-aminopropyltetrasiloxane. Add 4.24kg of N-β-aminobutyl-γ-aminopropyltetrasiloxane and 1.96kg of gluconic acid into reaction kettle 3, use ethanol as solvent, heat to 70°C, and react for 8 hours. Add 0.94 kg of propylene glycol diglycidyl ether again, and continue to react for 12 hours. The solvent ethanol is distilled off to obtain the tetrasiloxane gemini surfactant containing sugar amido group. It is measured that it does not hydrolyze within 6 months in water.
Embodiment 3
[0037] Add 16.24kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.54kg of concentrated sulfuric acid into reaction kettle 1, react at a temperature of 60°C for 4 hours, add sodium hydroxide to deactivate the catalyst, and filter The solid was removed and distilled under reduced pressure to obtain chloropropyltetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 6.12kg of 1,5-pentamethylenediamine into the reaction kettle 2, heat to 90°C, react for 2 hours, the mixture is allowed to stand for layers, and the upper layer is distilled under reduced pressure to obtain N-β -Aminoamyl-γ-aminopropyltetrasiloxane. Add 4.38 kg of N-β-aminopentyl-γ-aminopropyltetrasiloxane and 1.78 kg of mannolactone into reaction kettle 3, use methanol as solvent, heat to 80° C., and react for 10 hours. Then, 1.01 kg of butanediol diglycidyl ether was added, and the reaction was continued for 12 hours. The solvent methanol was distilled off to obtain a tetrasiloxane gemin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com