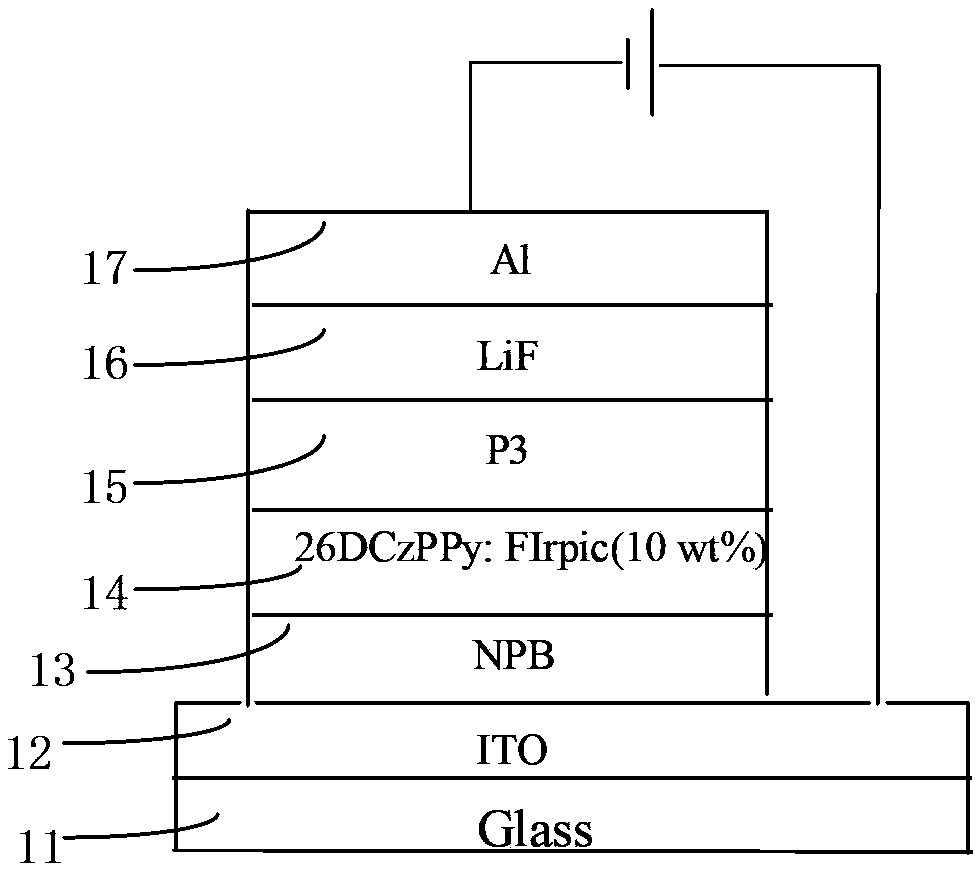
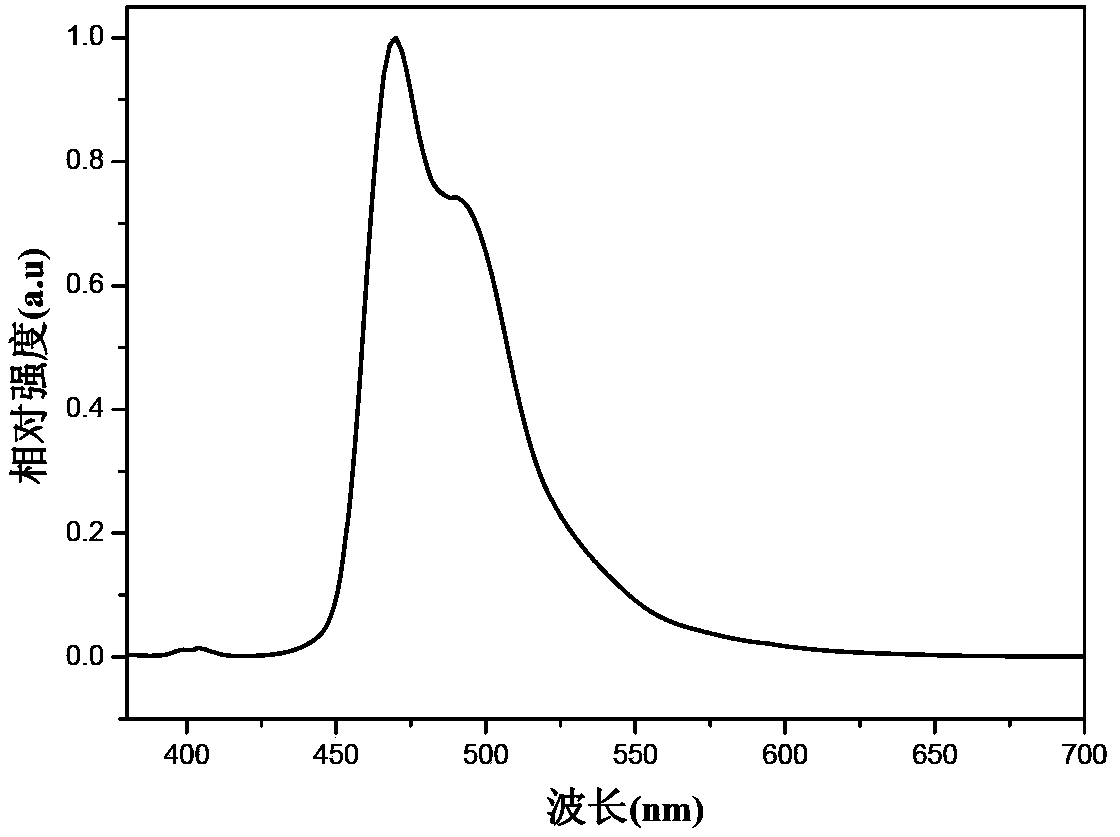
Electron transport material as well as preparation method and organic electroluminescence device thereof
An electron transport material and organic palladium technology, applied in the fields of organic electroluminescence devices, electron transport materials and their preparation, can solve the problems of lack of electron transport materials, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] This example provides a 2,2'-bis(5-trifluoromethylpyridin-2-yl)-9,9'-spirobifluorene (CF5PySPBF), whose chemical structure is shown in formula (2):
[0040]
[0041] The preparation steps of the above-mentioned CF5PySPBF are as follows:
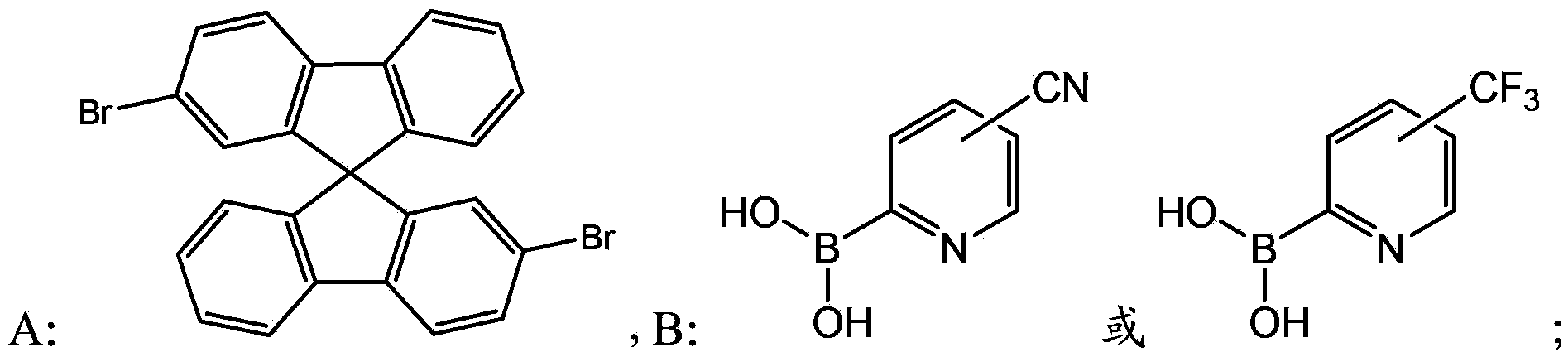
[0042] S10. Provide compound A (2,2,5-dibromo-9,95-spirobifluorene) and compound B1 ((5-trifluoromethylpyridin-2-yl)boronic acid) represented by the following structural formula:
[0043]
[0044] S20, Suzuki coupling reaction to prepare CF5PySPBF
[0045] Add 5.0mmol of A, 12.0mmol of B1 and 0.025mmol of tetrakis(triphenylphosphine) palladium into the reaction flask, vacuumize and circulate nitrogen for 3 times, make the reaction system in an oxygen-free state, and add 65ml of tetrahydrofuran solution and 40ml of Na 2 CO 3 (2mol / L) aqueous solution, reflux at 75-80°C, and Suzuki coupling reaction for 48 hours to obtain a reaction solution containing CF5PySPBF;
[0046] Separation and purification of S30 and CF5PySPBF
[004...
Embodiment 2
[0051] This example provides a 2,2'-bis(4-trifluoromethylpyridin-2-yl)-9,9'-spirobifluorene (CF4PySPBF), whose chemical structure is shown in formula (3):
[0052]
[0053] The preparation steps of the above-mentioned CF4PySPBF are as follows:
[0054] S10, providing compound A (2,2'-dibromo-9,9'-spirobifluorene) and compound B2 ((4-trifluoromethylpyridin-2-yl)boronic acid) represented by the following structural formula:
[0055]
[0056] S20, Suzuki coupling reaction to prepare CF4PySPBF
[0057] Add 5.0mmol of A, 10.0mmol of B2 and 0.15mmol of tris(dibenzylideneacetone) dipalladium to the reaction flask, vacuumize and circulate nitrogen for 3 times to make the reaction system in an anaerobic state, nitrogen protection Add 70ml of ethylene glycol dimethyl ether and 45ml of K 2 CO 3 (2mol / L) aqueous solution, reflux at 95°C, and Suzuki coupling reaction for 40 hours to prepare a reaction solution containing CF4PySPBF;
[0058] Separation and purification of S30 and CF...
Embodiment 3
[0063] This example provides a 2,2'-bis(6-cyanopyridin-2-yl)-9,9'-spirobifluorene (CN6PySPBF), whose chemical structure is shown in formula (4):
[0064]
[0065] The preparation steps of the above-mentioned CN6PySPBF are as follows:
[0066] S10, providing compound A (2,2'-dibromo-9,9-spirobifluorene) and compound B3 ((6-cyanopyridin-2-yl)boronic acid) represented by the following structural formula:
[0067]
[0068] S20, Suzuki coupling reaction to prepare CN6PySPBF
[0069] Add 5.0mmol of A, 11.0mmol of B3 and 0.5mmol of bis(triphenylphosphine)palladium dichloride into the reaction flask, vacuumize and circulate nitrogen for 3 times to make the reaction system in an oxygen-free state, nitrogen Add 60ml of toluene solution and 37.5ml of Cs under protection 2 CO 3 (2mol / L) aqueous solution, reflux at 120°C, and Suzuki coupling reaction for 24 hours to obtain a reaction solution containing CN6PySPBF;
[0070] Separation and purification of S30 and CN6PySPBF
[0071...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com