Application of schisandrol B in preparation of medicine for preventing and treating cholestatic liver disease
A technology of schisandra alcohol B and cholestasis, which is applied in the field of medicine to achieve the effects of reducing bile acid levels, reducing liver damage, and reducing the degree of liver tissue necrosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
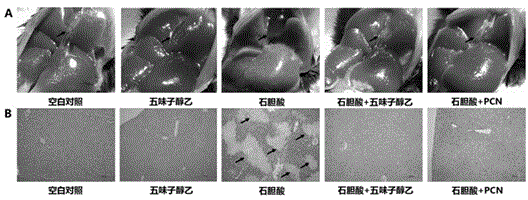
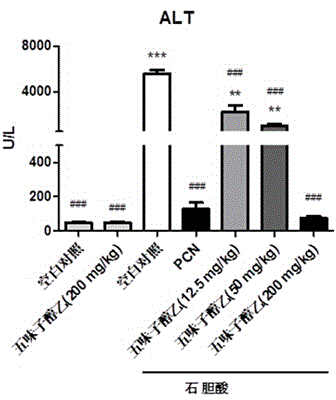
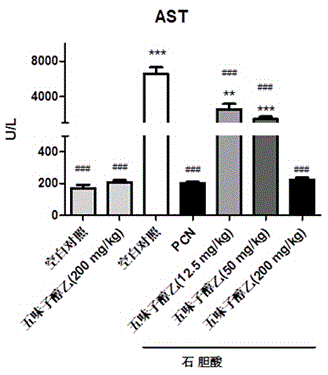
[0053] Example 1 Treatment of the mouse intrahepatic cholestasis model caused by excess lithocholic acid
[0054] 1. Materials and methods
[0055] (1) Main instruments:
[0056] 5417-R low-temperature high-speed centrifuge (Eppendorf, Germany); full-wavelength microplate reader (Thermo, USA); Mini-protein3 electrophoresis system (Bio-Rad, USA); Mini Trans-Blot transfer system (Bio-Rad, USA) ; ImageQuant LAS 4000 Exposure Imager (General Electric, USA); Gradient PCR (Eppendorf, Germany); 7500 Real Time PCR System (Applied Biosystems, USA).
[0057] (2) Drugs and reagents
[0058] Schisandra Alcohol B (purity ≥98%, Shanghai Ronghe Pharmaceutical Technology Development Co., Ltd.); lithocholic acid (purity ≥98%, Shanghai Aladdin Reagent Co., Ltd.), sodium carboxymethylcellulose (CMC, purity ≥98%, American Sigma Company); corn oil (pharmaceutical grade, Shanghai Aladdin Reagent Co., Ltd.); pregnaneenone 16-carbonitrile (PCN, purity ≥ 98%, American Sigma Company); ursodeoxycho...
Embodiment 2
[0097] Example 2 Treatment of extrahepatic cholestasis model in mice caused by bile duct ligation
[0098] 1. Materials and methods
[0099] (1) The main instruments are the same as in Example 1.
[0100] (2) Drugs and reagents: ursodeoxycholic acid (UDCA, Shanghai Aladdin Reagent Co., Ltd.); the rest are the same as in Example 1.
[0101] 2. Experimental animals and dosing regimen
[0102] C57BL / 6 mice, male, 8-9 weeks old, were provided by the Experimental Animal Center of Sun Yat-sen University (University Town, Guangzhou City), license number SCXK (Guangdong) 2011-0029, and normal mice were fed with maintenance feed.
[0103] Schisandrin B (200 mg / kg) was ultrasonically dispersed in pre-autoclaved 0.5% (w / w) CMC; ursodeoxycholic acid (25 mg / kg) was dissolved in saline.
[0104] Mice were randomly divided into 5 groups, 6 to 8 in each group, the groups were sham operation blank control group, sham operation + schisandrin B group, bile duct ligation blank control group,...
Embodiment 3
[0121] Example 3 Treatment of Schisandra Alcohol B to Cholesterol Stones, Hyperlipoproteinemia, etc.
[0122] The present invention has also preliminarily studied the impact and therapeutic effect of schisandra alcohol on cholesterol gallstones and hyperlipoproteinemia. The prevention and treatment effect is obvious.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com