Tri-block polymer micelle, preparation method and application
A polymer and tri-block technology, applied in the direction of drug combination, pharmaceutical formulation, genetic material components, etc., can solve problems such as difficult to degrade, high cytotoxicity, etc., to achieve enhanced penetration and retention, good biocompatibility and biological Effect of degradability and excellent tissue permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] This example provides a doxorubicin-loaded micelle of N-succinyl chitosan-poly-L-lysine-pallitic acid triblock polymer.
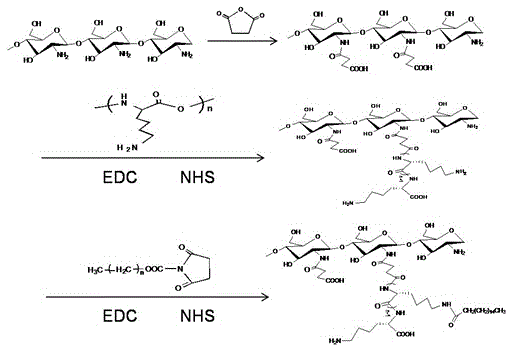
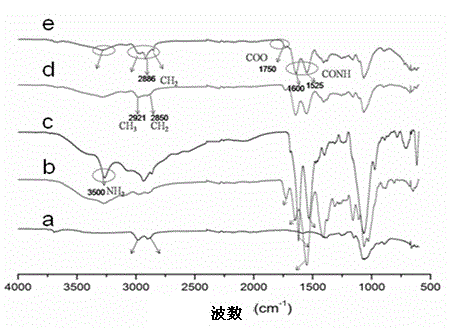
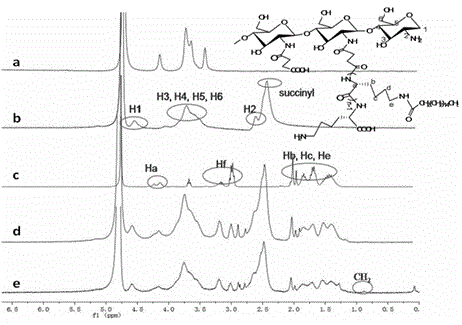
[0043] See attached figure 1 , which is a schematic diagram of the synthetic route of the N-succinyl chitosan-poly-L-lysine-soft fatty acid triblock polymer provided by the present invention. In this embodiment, the specific synthesis method includes the following steps:
[0044] 1. Synthesis of N-succinyl chitosan-poly-L-lysine (NSC-PLL)
[0045] Dissolve 0.4 g of NSC in 25 mL of distilled water and stir to form a solution. Put 0.32 g PLL, 0.192 g EDC·HCl and 0.057 g NHS into the above reaction solution respectively, 1.1 mol.L -1 Adjust pH to 5.6 with HcL, magnetically stir the reaction liquid, react at room temperature for 7 2 h, place in a dialysis bag (MWCO=3 500), dialyze with distilled water for 3 days, filter and freeze-dry to obtain NSC-PLL.
[0046] 2. Activation of palmitic acid (PA)
[0047] 0.128 g of soft fatty acid was added to 25...
Embodiment 2
[0058] This embodiment provides a kind of N-succinyl chitosan-poly-L-lysine-soft fatty acid triblock polymer loaded with siRNA micelles, the specific steps of preparation include: preparing N -Succinyl chitosan-poly-L-lysine-soft fatty acid triblock polymer, dissolve it in 5 ml deionized water to prepare 1 mg.mL -1 blank micellar solution. Incubate blank micelles with siRNA (N / P=20-40) at 37°C with shaking for 30 min to obtain N-succinyl chitosan-poly-L-lysine-soft fatty acid triblock polymer-loaded siRNA gel bundle.
[0059] The ability of the N-succinyl chitosan-poly-L-lysine-pallitic acid triblock polymer-loaded siRNA micelles to bind siRNA was determined by agarose gel electrophoresis. Add 17 μL DEPC-treated water, 1 μL, 2 μL, 4 μL, 8 μL, 16 μL micellar solution to 6 EP tubes of RNase, and make up to 18 μL for each group with DEPC-treated water . Mix the solutions in each EP tube thoroughly and let stand at room temperature for 30 min. Take 15 μL and add 3 μL 6×loading...
Embodiment 3
[0063] In this example, N-succinyl chitosan-poly-L-lysine-pallitic acid triblock polymer was prepared to co-transport doxorubicin and siRNA micelles. The N-succinyl chitosan-poly-L-lysine-soft fatty acid triblock polymer prepared by the technical scheme of Example 1 was dissolved in 5 ml deionized water to obtain 1 mg.mL -1 The blank micelle solution of the doxorubicin hydrochloride was dissolved with an appropriate amount of DMSO solution, and triethylamine with a molar ratio of 1:2 to the doxorubicin hydrochloride was added for desalting treatment, and then the desalted doxorubicin was slowly added dropwise to the above blank In the micellar solution, the final concentration of the drug was 0.2 mg.mL -1 ; Sonication for 30 min, magnetic stirring for 2 h, and then placed in a dialysis bag for dialysis for 4 h, changing distilled water every 1 h, and filtering the dialysate with a 0.45 μm microporous membrane to obtain a doxorubicin-loaded micelle sample. Incubate drug-loaded...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Viscosity average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com