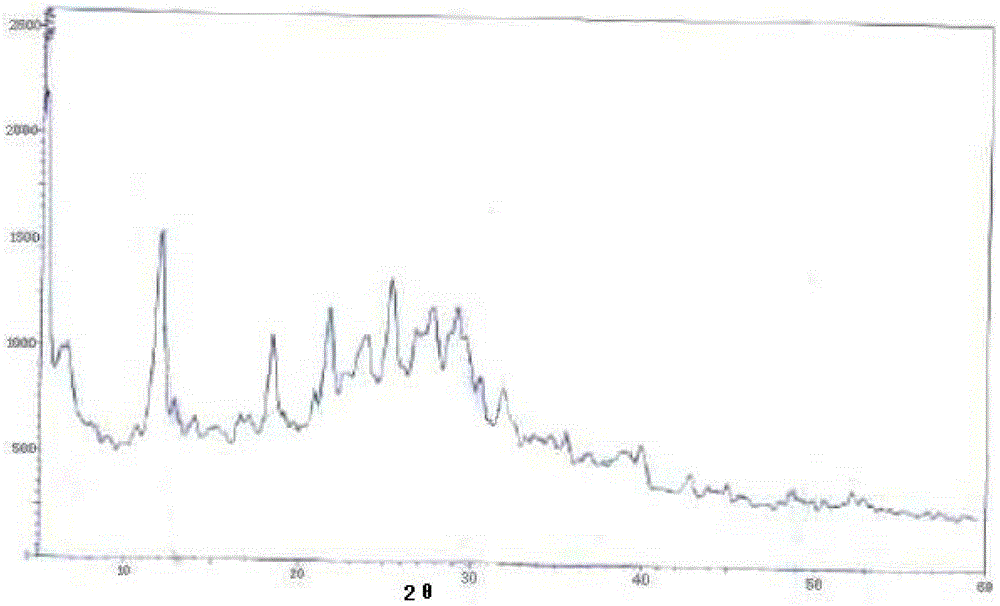
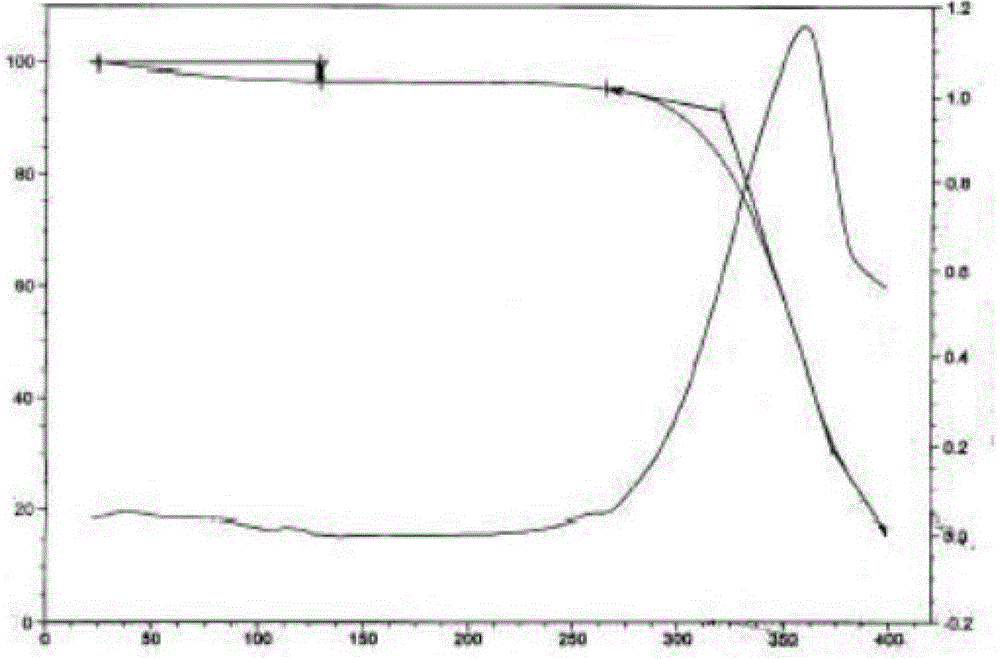
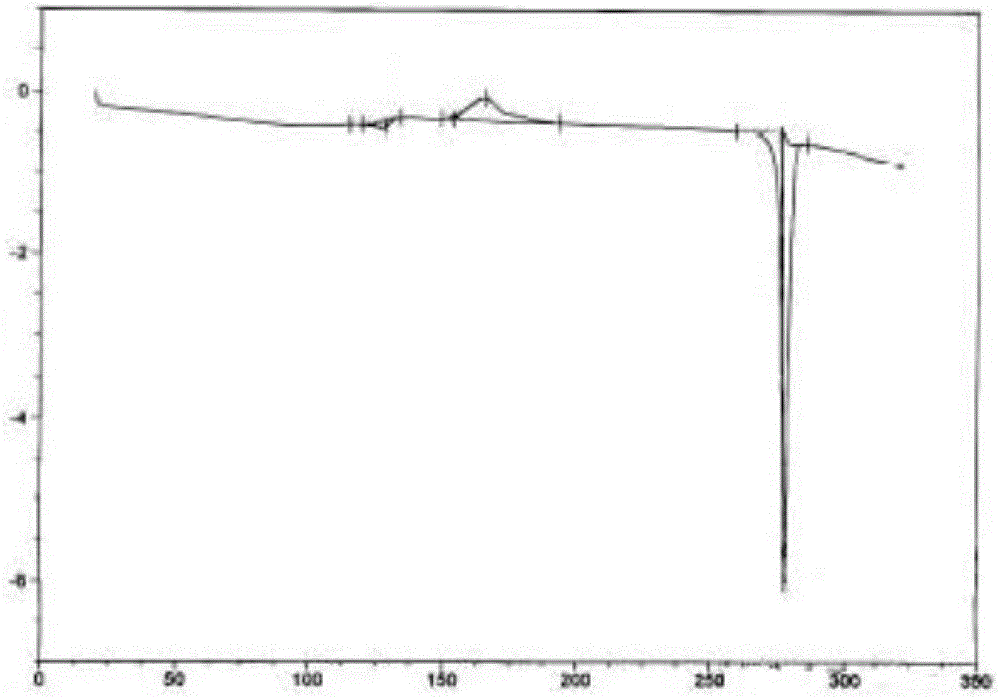
Epinastine hydrochloride crystal form, and preparation method and application thereof
A kind of epilastine hydrochloride crystal and epilastine hydrochloride technology, which are applied in the field of epilastine hydrochloride crystal form and its preparation and use, and can solve the problems of decreased drug stability, decreased drug effect, precipitation of liquid preparations and the like , to achieve the effect of improving stability and solubility, facilitating large-scale production and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Embodiment 1 The preparation method of epinastine hydrochloride crystal form IX of the present invention
[0078] Weigh 2.0g of epinastine hydrochloride, dissolve it with 3mL of water / acetone mixed solution (water:acetone=2:1) at an internal temperature of 60±5°C, add 0.1g of activated carbon, stir for decolorization, and then seal it and transfer it to a clean area , filtered while hot, cooled and crystallized, when a large amount of crystals precipitated, stood at -5~0°C for 8±1h, collected the crystals, and dried under reduced pressure to obtain 1.990g of epinastine hydrochloride crystal form IX, with a yield of 99.5%, melting point 275.6°C-275.9°C.
Embodiment 2
[0079] Example 2 The preparation method of epinastine hydrochloride crystal form IX of the present invention
[0080] Weigh 2.0g of epinastine hydrochloride, dissolve it with 2mL of water / acetone mixed solution (water:acetone=1:1) at an internal temperature of 60±5°C, add 0.1g of activated carbon, stir to decolorize, and then seal it and transfer it to a clean area , filtered while hot, cooled and crystallized, and when a large amount of crystals precipitated, they were left standing at -5~0°C for 8±1h, the crystals were collected, and dried under reduced pressure to obtain 1.986g of epinastine hydrochloride crystal form IX, with a yield of 99.3%, melting point 275.5°C-275.9°C.
Embodiment 3
[0081] Example 3 The preparation method of epinastine hydrochloride crystal form IX of the present invention
[0082] Weigh 2.0g of epinastine hydrochloride, dissolve it with 10mL of dichloromethane / ethanol mixed solution (dichloromethane:ethanol=4:1) at an internal temperature of 60±5°C, add 0.1g of activated carbon, stir to decolorize, and then seal it tightly Transfer to a clean area, filter while it is hot, cool and crystallize, and when a large amount of crystals precipitate, stand still at -5~0°C for 8±1h, collect the crystals, and dry under reduced pressure to obtain 1.976g of epinastine hydrochloride crystal form IX , the yield is 98.8%, and the melting point is 275.6°C-275.9°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com