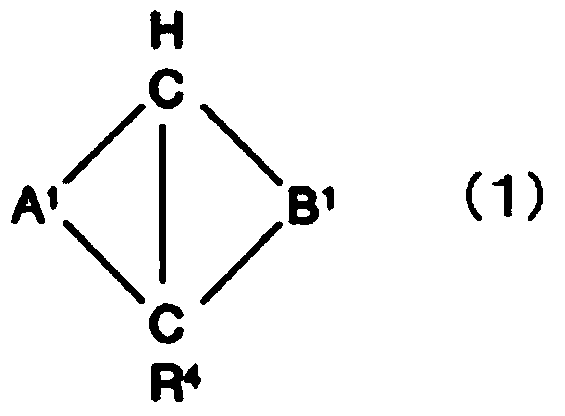
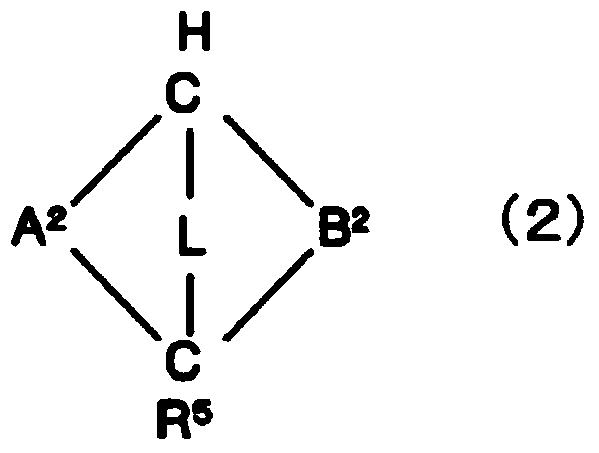
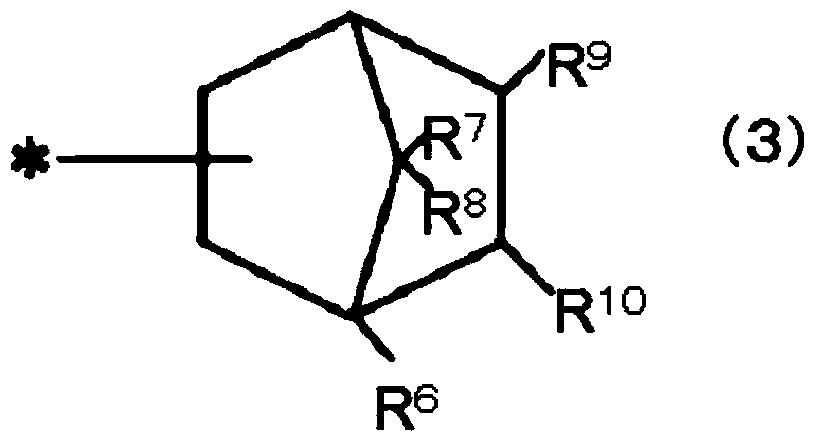
(METH)acrylate-based polymer, composition comprising same and use thereof
A technology of acrylate and polymer, which is applied in the photoplate-making process of the pattern surface, photosensitive materials used in photomechanical equipment, instruments, etc., can solve the problems of insufficient dye brightness and achieve excellent dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0148] Into a flask equipped with a stirring device, a dropping funnel, a condenser, a thermometer, and a gas introduction tube, 262.0 g of propylene glycol monomethyl ether acetate was charged, stirred while nitrogen substitution was performed, and the temperature was raised to 120°C.
[0149] Next, 49.7 g (0.27 moles) of 2-ethylhexyl acrylate, 99.4 g (0.7 moles) of glycidyl methacrylate, and 2-ethylhexyl acrylate with a glass transition temperature of -70°C when forming a homopolymer, and To a monomer mixture of 6.6 g (0.03 mol) of cyclopentyl ester, 19.0 g of tert-butyl peroxy-2-ethylhexanoate (polymerization initiator, manufactured by NOF Corporation, Parbull O) was added, and the obtained substance It was added dropwise to the above-mentioned flask from the dropping funnel over 2 hours. After completion of the dropwise addition, the mixture was further stirred at 120° C. for 2 hours to perform a copolymerization reaction to produce an addition copolymer (precursor of a cu...
Embodiment 2
[0152] In place of 2-ethylhexyl acrylate, 130.1 g (0.27 mol) of methoxypolyethylene glycol acrylate (AM-90G manufactured by Shin-Nakamura Chemical Industry Co., Ltd.) with a glass transition temperature of -71°C when forming a homopolymer was used 49.7 g (0.27 mol) and 19.0 g of tert-butyl peroxy-2-ethylhexanoate were changed to 28.8 g, and the curable polymer (B) was obtained like Example 1. The Tg of the polymer is -9°C, the acid value is 23KOHmg / g, the unsaturated group equivalent is 480g / mol, and the weight average molecular weight is 8700. In addition, the hydroxyl value was 93KOHmg / g. Next, propylene glycol monomethyl ether was added to the reaction solution to prepare a polymer solution having a solid content concentration of the curable polymer (B) of 40%. Let this be sample 2.
Embodiment 3
[0154]In addition to using 94.0 g (0.37 moles) of m-phenoxybenzyl methacrylate with a glass transition temperature of -35 °C when forming a homopolymer instead of 49.7 g (0.27 moles) of 2-ethylhexyl acrylate, and adding 99.4 g (0.7 mol) of glycidyl acrylate was changed to 85.2 g (0.6 mol), tert-butyl peroxy-2-ethylhexanoate was changed to 22.7 g, 50.4 g (0.7 mol) of acrylic acid was changed to 43.2 g Except that (0.6 mol), the curable polymer (C) was obtained in the same manner as in Example 1. The Tg of the polymer is -8°C, the acid value is 29KOHmg / g, the unsaturated group equivalent is 460g / mol, and the weight average molecular weight is 7200. In addition, the hydroxyl value was 94KOHmg / g. Next, propylene glycol monomethyl ether was added to the reaction solution to prepare a polymer solution having a solid content concentration of the curable polymer (B) of 40%. Let this be sample 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Acid value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com