Placental growth factor loaded nano-particle, as well as preparation method and application thereof
A technology of placental growth factor and nanoparticle, which is applied in the direction of pharmaceutical formulations, medical preparations containing no active ingredients, and medical preparations containing active ingredients. It can solve the problems of good slow-release effect and high encapsulation rate, and achieve slow-release Good effect, good stability, and not easy to protein denaturation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
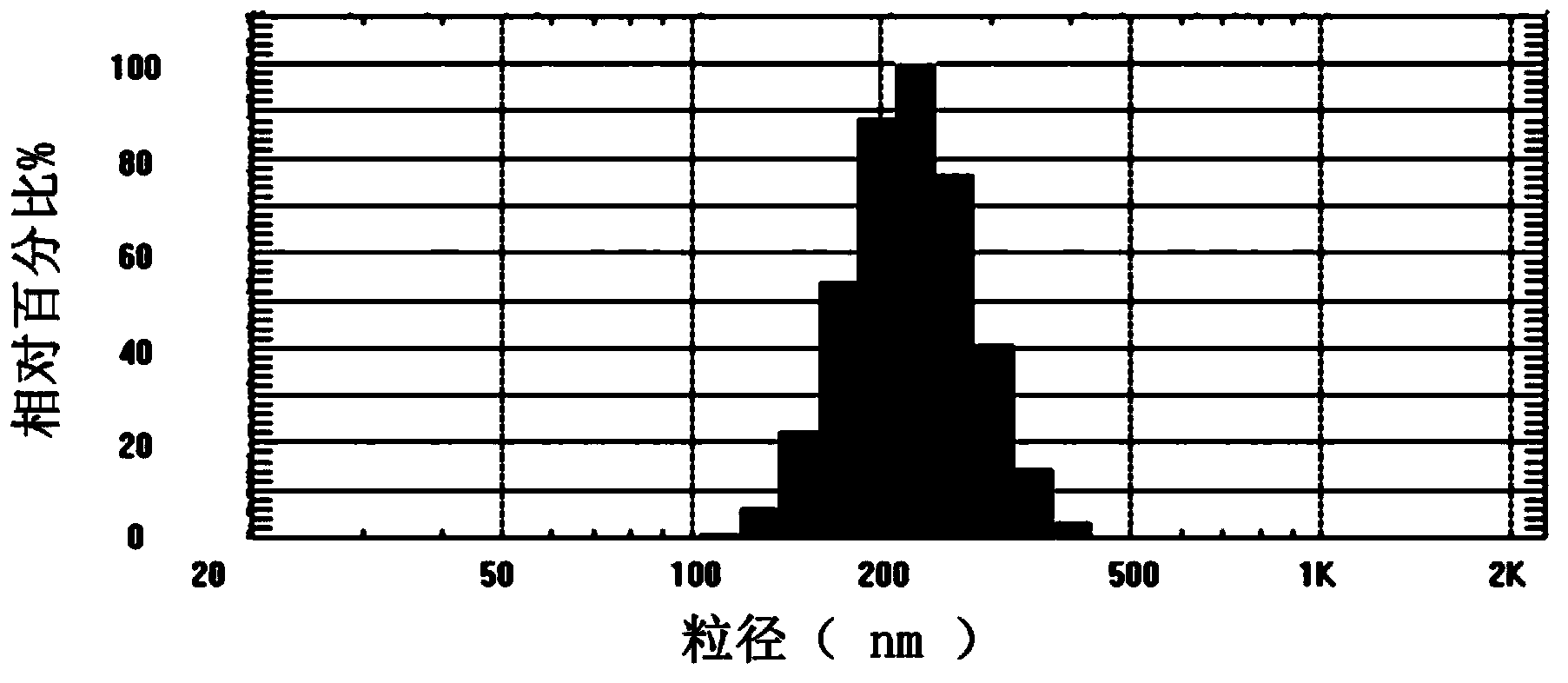
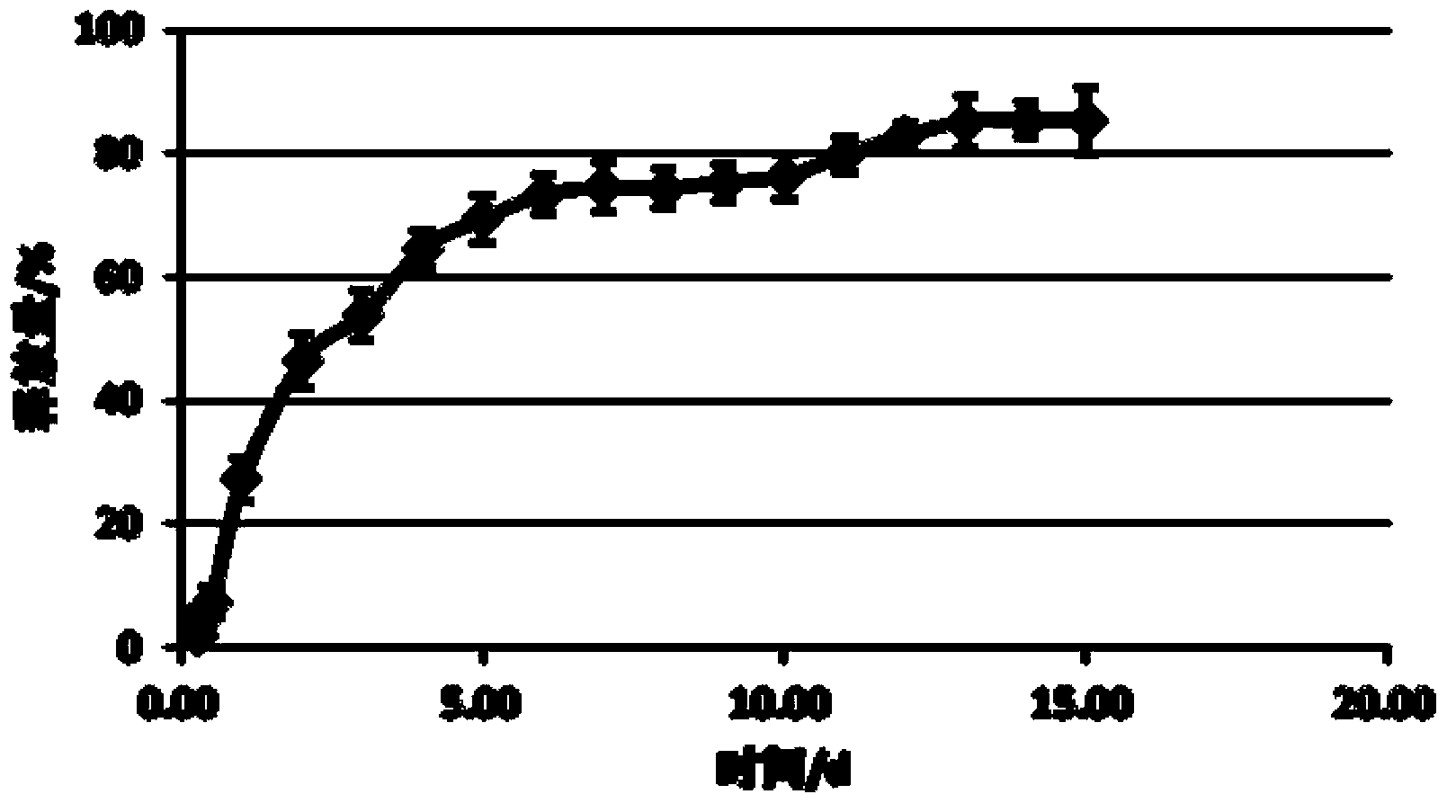
Image
Examples
Embodiment 1
[0052] (1) Preparation of the inner water phase: prepare 0.15 mg / mL PLGF aqueous solution, take 100 μL (0.1 mL) as the inner water phase (W1), and the concentration is the mass ratio of the placental growth factor to the volume of the inner water phase.
[0053] (2) Preparation of the oil phase: Dissolve 10 mg of PLGA (viscosity average molecular weight 60,000, lactic acid, glycolic acid block ratio 50:50) in 0.5 mL of ethyl acetate to prepare a 20 mg / mL solution as the oil phase ( O); said percentage is the mass of PLGA and the volume ratio of ethyl acetate.
[0054] (3) Add W1 dropwise to O, and ultrasonically disperse. The power of ultrasonic dispersion is 200w, and the time is 2min, forming a water-in-oil (W1 / O) emulsion.
[0055] (4) Add the (W1 / O) type emulsion in step (3) to 2mL of 19% Pluronic F-68 aqueous solution (i.e. the outer aqueous phase), the percentage is Pluronic F-68 The weight percent of the external water phase; under ice bath conditions, use a dispersing...
Embodiment 2
[0061] (1) Preparation of the inner water phase: prepare 0.15 mg / mL PLGF aqueous solution, take 100 μL as the inner water phase (W1), and the stated concentration is the mass ratio of the placental growth factor to the volume of the inner water phase.
[0062] (2) Preparation of the oil phase: Dissolve 5 mg of PLGA (viscosity average molecular weight 60,000, lactic acid, glycolic acid block ratio 50:50) in 0.5 mL of ethyl acetate, and prepare a 10 mg / mL solution as the oil phase (O); said percentage is the volume ratio of the mass of PLGA to ethyl acetate.
[0063] (3) Slowly add W1 to O, and ultrasonically disperse. The power of ultrasonic dispersion is 200w, and the time is 1min, forming a water-in-oil (W1 / O) emulsion.
[0064] (4) Add the (W1 / O) type emulsion in step (3) to 2mL of 15% Pluronic F-68 aqueous solution (i.e. the outer aqueous phase), the percentage is Pluronic F-68 in the outer The weight percent of the water phase; under ice bath conditions, the high-speed sh...
Embodiment 3
[0069] (1) Preparation of the inner water phase: prepare 0.20 mg / mL PLGF aqueous solution, take 100 μL as the inner water phase (W1), and the concentration is the mass ratio of the placental growth factor to the inner water phase.
[0070] (2) Preparation of the oil phase: Dissolve 10 mg of PLGA (viscosity average molecular weight 60,000, lactic acid, glycolic acid block ratio 50:50) in 0.5 mL of ethyl acetate to prepare a 20 mg / mL solution as the oil phase (O); said percentage is the volume ratio of the mass of PLGA to ethyl acetate.
[0071] (3) Slowly add W1 to O, and ultrasonically disperse. The power of ultrasonic dispersion is 200w, and the time is 1min, forming a water-in-oil (W1 / O) emulsion.
[0072] (4) Add the (W1 / O) type emulsion in step (3) to 3mL of 10% Pluronic F-68 aqueous solution (i.e. the outer aqueous phase), the percentage is Pluronic F-68 in the outer The weight percent of the water phase; under ice bath conditions, the high-speed shear emulsification of ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com