Preparation method of anti-thyroid ointment for external application
An anti-thyroid and ointment technology, applied in ointment delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 8
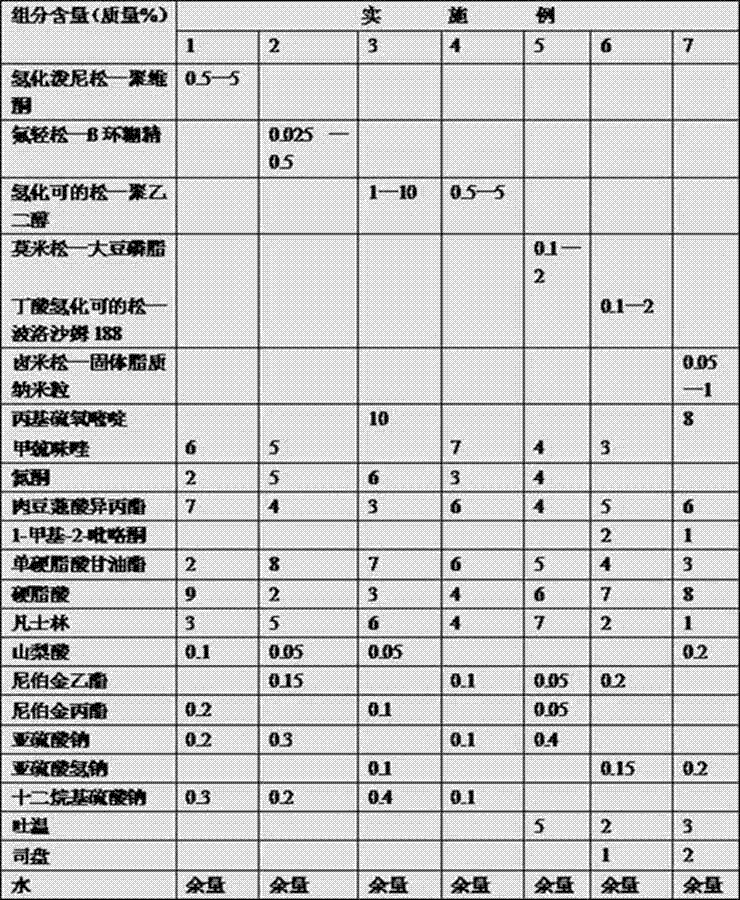
[0049] Example 8. This example adopts the formula of implementing 1 to prepare
[0050] (1) Put the original drug of prednisolone and 10 times (mass ratio) of povidone in 50 times (mass ratio) of ethanol, stir and dissolve on a constant temperature water bath at 60°C, evaporate the solvent, collect the solids, and dry Finally, pulverize and pass through an 80-mesh sieve to obtain prednisone-povidone solid dispersion powder.
[0051] (2) Put antithyroid drugs, preservatives, antioxidants and emulsifiers into distilled water and mix well, heat to 80°C, mix well to obtain the water phase;
[0052] (3) Heat the oil-soluble matrix and penetration enhancer to 80°C to melt, and mix well to obtain the oil phase;
[0053] (4) Pour the oil phase obtained in step (3) into the water phase obtained in step (2), heat to 80°C, and stir evenly;
[0054] (5) When the temperature drops to 40°C, add the prednisolone-povidone solid dispersion powder prepared in step (1);
[0055] (6) Stir full...
example 9
[0057] Example 9. This example adopts the formula of implementing 2 to prepare
[0058] (1) Weigh 8.0 g of β-cyclodextrin, add 100 ml of water, and heat to 61°C to make a saturated solution. Add the original drug of fluocinolone to 50 times boiling ethanol to dissolve, then slowly add it dropwise to the cyclodextrin aqueous solution which is equimolar to the drug, and stir at constant temperature for an appropriate time. Place in a -4°C refrigerator to precipitate the solid, and filter after 24 h. First wash the precipitated solid with a small amount of ethanol for 3 times, then wash with distilled water for 3 times, drain and dry at 60°C to obtain the prednisolone-β-cyclodextrin inclusion compound;
[0059] (2) Put antithyroid drugs, preservatives, antioxidants and emulsifiers into distilled water and mix well, heat to 80°C, mix well to obtain the water phase;
[0060] (3) Heat the oil-soluble matrix and penetration enhancer to 80°C to melt, and mix well to obtain the oil p...
Embodiment 10
[0065] Embodiment 10, this example adopts the formula of implementing 3 to prepare
[0066] (1) Dissolve the former drug of hydrocortisone in 30 times (mass ratio) of boiling ethanol. Then heat and melt 10 times (mass ratio) polyethylene glycol (4000) of hydrocortisone in a water bath, then mix the two liquids evenly, evaporate the solvent under constant stirring, and then transfer to an ice-water bath to cool and solidify. Move it to a desiccator, dry it in vacuum, make it embrittled, and pulverize it to obtain the hydrocortisone-polyethylene glycol solid dispersion;
[0067] (2) Put antithyroid drugs, preservatives, antioxidants and emulsifiers into distilled water and mix well, heat to 80°C, mix well to obtain the water phase;
[0068] (3) Heat the oil-soluble matrix and the penetration enhancer to 80°C to melt, and mix well to obtain the oil phase;
[0069] (4) Pour the oil phase obtained in step (3) into the water phase obtained in step (2), heat to 80°C, and stir evenl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com