Method for producing industrial-grade magnesium sulfate by using titanium white waste acid
A technology of titanium dioxide waste acid and magnesium sulfate, which is applied in magnesium sulfate and other directions, can solve the problems of difficulty in coordinating the quality and production cost of magnesium sulfate products, greatly reducing the content of magnesium sulfate, and increasing the solid content of acid hydrolysis slurry, so as to reduce the Processing difficulty, significant economic benefits, and the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
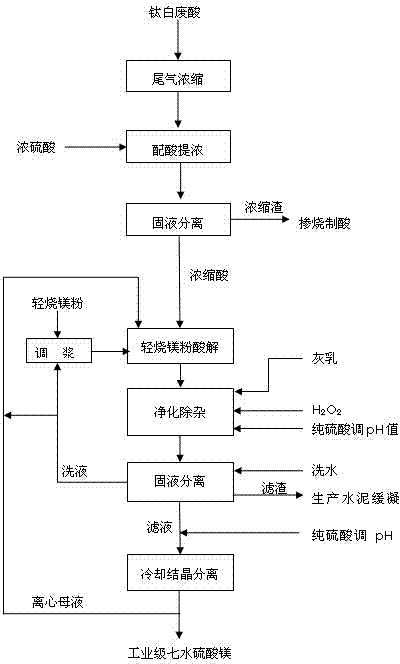
[0028] Titanium dioxide waste acid, its main component, is calculated by mass ratio as: H 2 SO 4 20.11%, FeSO 4 8.78%, Al 2 (SO4) 3 0.57% MgSO 4 2.07%, TiO 2 0.34%, after countercurrent spraying and rotary kiln calcination tail gas, use the waste heat of calcination tail gas to enrich to H 2 SO 4 28%, and then recover metatitanic acid by waste acid flocculation and sedimentation to obtain clear waste acid. Add the clear waste acid to the waste acid preparation tank, and slowly add concentrated sulfuric acid with a sulfuric acid mass fraction of 98% under stirring conditions, add 1.99t concentrated sulfuric acid for every 5t of 28% waste acid, and mix the two evenly to obtain a mixed acid. Then slowly cool down and mature, the gradient cooling range is 8°C / h, the end point temperature is 60°C, and after aging for 1 hour, all the mixed acid is poured into a filter press for solid-liquid separation, and concentrated acid is obtained after separation, with a mass f...
Embodiment 2
[0030] The waste acid in the production process of titanium dioxide, its main component, is calculated by mass ratio as: H 2 SO 4 20.11%, FeSO 4 8.78%, Al 2 (SO4) 3 0.57% MgSO 4 2.07%, TiO 2 0.34%, after countercurrent spraying and rotary kiln calcination tail gas, use the waste heat of calcination tail gas to enrich to H 2 SO 4 28%, and then recover metatitanic acid by waste acid flocculation and sedimentation to obtain clear waste acid. Add the clear waste acid to the waste acid preparation tank, slowly add concentrated sulfuric acid with a sulfuric acid mass fraction of 98% under stirring conditions, add 1.53t concentrated sulfuric acid for every 5t of 28% waste acid, and mix the two evenly to obtain a mixed acid. Then carry out slow cooling and cooling, aging, the range of gradient cooling is 8°C / h, the end point temperature is 60°C, and after aging for 1 hour, all the mixed acid is poured into a filter press for solid-liquid separation, and the concentrate...
Embodiment 3
[0032] The waste acid in the production process of titanium dioxide, its main component, is calculated by mass ratio as: H 2 SO 4 20.11%, FeSO 4 8.78%, Al 2 (SO4) 3 0.57% MgSO 4 2.07%, TiO 2 0.34%, after countercurrent spraying and rotary kiln calcination tail gas, use the waste heat of calcination tail gas to enrich to H 2 SO 4 28%, and then recover metatitanic acid by waste acid flocculation and sedimentation to obtain clear waste acid. Add the clear waste acid to the waste acid preparation tank, and slowly add concentrated sulfuric acid with a sulfuric acid mass fraction of 98% under stirring conditions, add 1.99t concentrated sulfuric acid for every 5t of 28% waste acid, and mix the two evenly to obtain a mixed acid. Then slowly cool down and mature, the gradient cooling range is 8°C / h, the end point temperature is 60°C, and after aging for 1 hour, all the mixed acid is poured into a filter press for solid-liquid separation, and concentrated acid is obtaine...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com